Abstract
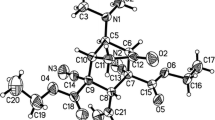
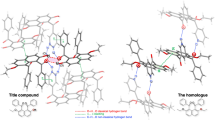
Detailed study of the nature of weak coordination K-O interactions and strong O-H···O hydrogen bonds and estimation of their energy in a potassium hydrophthalate crystal have been performed on the basis of the topological analysis of the electron density distribution function, obtained from precise X-ray diffraction study at 100 K (R1 = 0.0110), within Bader’s “Atoms in Molecule” theory. It is shown that the charge transfer accompanying the formation of cation···anion associates in the crystal is only 0.05 e.
Similar content being viewed by others
References
E. A. Meyer, R. K. Castellano, and F. Diederich, Angew. Chem., Int. Ed. Engl. 42, 1210 (2003).
L. Brammer, Chem. Soc. Rev. 33, 476 (2004).
D. Braga, L. Brammer, and N. R. Champness, Cryst. Eng. Commun. 7, 1 (2005).
S. Kitagawa, R. Kitaura, and S. Noro, Angew. Chem., Int. Ed. Engl. 43, 1466 (2004).
C. N. R. Rao, S. Natarajan, and R. Vaidhyanathan, Angew. Chem., Int. Ed. Engl. 43, 2334 (2004).
C. V. Krishnamohan Sharma, Cryst. Growth Des. 6, 465 (2002).
L. Perez-Garcia and D. B. Amabilino, Chem. Soc. Rev. 31, 342 (2002).
Yu. V. Nelyubina, K. A. Lysenko, A. N. Kravchenko, et al., Izv. Akad. Ros. Nauk, Ser. Khim. 3, 387 (2006).
D. A. Lenev, K. A. Lyssenko, and R. G. Kostyanovsky, Eur. J. Inorg. Chem., 2979 (2003).
I. Corral, O. Mó, M. Yáñez, and L. Radom, J. Phys. Chem. A 109, 6735 (2005).
T. S. Koritsanszky and P. Coppens, Chem. Rev. 101, 1583 (2001).
C. Gatti, Z. Kristallogr. 220, 399 (2005).
V. G. Tsirelson and R. P. Ozerov, Electron Density and Bonding in Crystals: Principles, Theory and X-ray Diffraction Experiments in Solid State Physics and Chemistry (IOP, Bristol, 1996).
R. F. W. Bader, Atoms in Molecules. A Quantum Theory (Clarendon, Oxford, 1990; Mir, Moscow, 2001).
E. Espinosa, E. Molins, and C. Lecomte, Chem. Phys. Lett. 285, 170 (1998).
E. Espinosa, I. Alkorta, I. Rozas, et al., Chem. Phys. Lett. 336, 457 (2001).
K. A. Lyssenko, A. A. Korlyukov, D. G. Golovanov, et al., J. Phys. Chem. A 110, 6545 (2006).
I. V. Glukhov, K. A. Lysenko, A. A. Korlyukov, and M. Yu. Antipin, Izv. Ros. Akad. Nauk, Ser. Khim. 3, 541 (2005).
E. A. Zhurova, V. G. Tsirelson, A. I. Stash, et al., J. Chem. Phys. B 108, 20173 (2004).
K. A. Lyssenko, Yu. V. Nelyubina, R. G. Kostyanovsky, and M. Yu. Antipin, Chem. Phys. Chem. 7, 2453 (2006).
E. A. Pidko and R. A. van Santen, Chem. Phys. Chem. 7, 1657 (2006).
S. M. Harte, A. Parkin, A. Goeta, and C. C. Wilson, J. Mol. Struct. 741, 9 (2005).
A. Cavalli, P. Carloni, and M. Recanatini, Chem. Rev. 106, 3497 (2006).
G. N. Vayssilov, J. A. Lercher, and N. Rösch, J. Phys. Chem. B 104, 8614 (2000).
D. F. Plant, A. Simperler, and R. G. Bell, J. Phys. Chem. B 110, 6170 (2006).
N. Kejalakshmy and K. Srinivasan, J. Phys., D. Appl. Phys. 36, 1778 (2003).
G. M. Sheldrick, SHELXTL PLUS. Version 5.10 (Bruker AXS Inc., Madison, WI, USA).
N. K. Hansen and P. Koppens, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr. 34, 909 (1978).
T. S. Koritsansky, S. T. Howard, T. Richter, et al., XD-A Computer Program Package for Multipole Refinement and Topological Analysis of Charge Densities from Diffraction Data (2003).
F. L. Hirshfeld, Acta. Crystallogr. 32, 239 (1976).
D. A. Kirzhnits, Yu. E. Lozovik, and G. V. Shpatakovskaya, Usp. Fiz. Nauk 711, 3 (1975).
A. Stash and V. Tsirelson, WinXPRO. Program for Calculation of the Crystal and Molecular Propeties Using the Model Electron Density (Moscow, 2001).
K. A. Lysenko and M. Yu. Antipin, Izv. Ros. Akad. Nauk, Ser. Khim. 1, 1 (2006).
G. V. Gibbs, M. A. Spackman, D. Jayatilaka, et al., J. Phys. Chem. A 110, 12259 (2006).
R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr. 32, 751 (1976).
M. Meot-Ner, Chem. Rev. 105, 213 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.V. Nelyubina, K.A. Lyssenko, M.Yu. Antipin, 2008, published in Kristallografiya, 2008, Vol. 53, No. 2, pp. 219–225.
Rights and permissions
About this article
Cite this article
Nelyubina, Y.V., Lyssenko, K.A. & Antipin, M.Y. Estimation of the energy of coordination K-O bonds in a potassium hydrophthalate crystal on the basis of electron-density distribution analysis. Crystallogr. Rep. 53, 192–198 (2008). https://doi.org/10.1134/S1063774508020053
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774508020053