Abstract
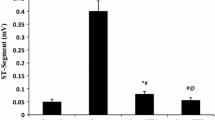
The antidiabetic drug empagliflozin is reported to have many cardioprotective effects. However, no studies have investigated the protective effects of empagliflozin (EMPA) in isoprenaline (ISO)-induced cardiac oxidative damage-a model mimicking the harmful effects of excess catecholamines on the heart. Therefore, in this study, we aimed to reveal the protective effect of EMPA in isoproterenol ISO-induced myocardial infarction in rats. We induced myocardial infarction by subcutaneously injecting ISO (100 mg/kg). To determine the protective effects of EMPA on the myocardial damage, we administered two different doses (10 and 20 mg/kg) by gavage for 14 days. Here we have shown that a 20 mg/kg dose of EMPA completely rescues rats from myocardial infarction by normalizing the following: elevated ST-segment, increased heart rate, decreased R amplitude, prolongation of the QT interval, and shortened RR interval. In addition, EMPA (20 mg/kg) ameliorates ISO-induced changes in serum cTnI, CK, ischemia-modified albumin (IMA), LDH, AST, ALT levels, and heart index. It improves serum lipid profile by decreasing cholesterol, triglycerides, LDL, and VLDL levels, and by increasing HDL levels. Moreover, EMPA (20 mg/kg) alleviates increased myocardial oxidative stress and inflammation by decreasing MDA, TNF-α, and IL-6 levels and increasing SOD and GPx levels. Furthermore, 20 mg/kg EMPA leads to reductions in DNA damage and apoptosis by downregulating of 8-OHdG and caspase-3 expressions. Collectively, EMPA exerts its protective effects on myocardial damage by improving oxidative stress, apoptosis, lipid profile and oxidative DNA damage in ISO-induced experimental myocardial infarction in rats.







Similar content being viewed by others
REFERENCES
Abdelzaher, W.Y., Ahmed, S.M., Welson, N.N., Alsharif, K.F., Batiha, G.E.S., and Labib, D.A.A., Dapsone ameliorates isoproterenol-induced myocardial infarction via Nrf2/ HO-1; TLR4/TNF-α signaling pathways and the suppression of oxidative stress, inflammation, and apoptosis in rats, Front. Pharmacol., 2021, vol. 12, p. 669679.
Andreadou, I., Efentakis, P., Balafas, E., Togliatto, G., Davos, C.H., Varela, A., Dimitriou, C.A., Nikolaou, P.-E., Maratou, E., Lambadiari, V., Ikonomidis, I., Kostomitsopoulos, N., Brizzi, M.F., Dimitriadis, G., and Iliodromitis, E.K., Empagliflozin limits myocardial infarction in vivo and cell death in vitro: role of STAT3, mitochondria, and redox aspects, Front. Physiol., 2017, vol. 8, p. 1077.
Ashrafi Jigheh, Z., Ghorbani Haghjo, A., Argani, H., Roshangar, L., Rashtchizadeh, N., Sanajou, D., Nazari Soltan Ahmad, S., Rashedi, J., Dastmalchi, S., and Mesgari Abbasi, M., Empagliflozin alleviates renal inflammation and oxidative stress in streptozotocin-induced diabetic rats partly by repressing HMGB1-TLR4 receptor axis, Iran. J. Basic Med. Sci., 2019, vol. 22, pp. 384–390.
Baartscheer, A., Schumacher, C.A., Wüst, R.C.I., Fiolet, J.W.T., Stienen, G.J.M., Coronel, R., and Zuurbier, C.J., Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits, Diabetologia, 2017, vol. 60, pp. 568–573.
Çetin, E., Pretreatment with β-glucan attenuates isoprenaline-induced myocardial injury in rats, Exp. Physiol., 2019, vol. 104, pp. 505–513.
Chawla, R., Goyal, N., Calton, R., and Goyal, S., Ischemia modified albumin: a novel marker for acute coronary syndrome, Indian J. Clin. Biochem., 2006, vol. 21, pp. 77–82.
Dhalla, N.S., Adameova, A., and Kaur, M., Role of catecholamine oxidation in sudden cardiac death, Fundam. Clin. Pharmacol., 2010, vol. 24, pp. 539–546.
El-Gohary, O.A. and Allam, M.M., Effect of vitamin D on isoprenaline-induced myocardial infarction in rats: possible role of peroxisome proliferator-activated receptor-γ, Can. J. Physiol. Pharmacol., 2017, vol. 95, pp. 641–646.
Ellison, G.M., Torella, D., Karakikes, I., Purushothaman, S., Curcio, A., Gasparri, C., Indolfi, C., Cable, N.T., Goldspink, D.F., and Nadal-Ginard, B., Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells, J. Biol. Chem., 2007, vol. 282, pp. 11397–11409.
Ertekin, B., Kocak, S., Defne Dundar, Z., Girisgin, S., Cander, B., Gul, M., Doseyici, S., Mehmetoglu, I., and Kemal Sahin, T., Diagnostic value of ischemia-modified albumin in acute coronary syndrome and acute ischemic stroke, Pak. J. Med. Sci., 2013, vol. 29, pp. 1003–1007.
Fala, L., Jardiance (Empagliflozin), an SGLT2 inhibitor, receives FDA approval for the treatment of patients with type 2 diabetes, Am. Health Drug Benefits, 2015, vol. 8, pp. 92–95.
Farvin, K.H.S., Anandan, R., Kumar, S.H.S., Shiny, K.S., Mathew, S., Sankar, T. V, and Nair, P.G.V., Cardioprotective effect of squalene on lipid profile in isoprenaline-induced myocardial infarction in rats, J. Med. Food, 2006, vol. 9, pp. 531–536.
Fiordaliso, F., Cuccovillo, I., Bianchi, R., Bai, A., Doni, M., Salio, M., De Angelis, N., Ghezzi, P., Latini, R., and Masson, S., Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes, Life Sci., 2006, vol. 79, pp. 121–129.
Forte, E., Panahi, M., Baxan, N., Ng, F.S., Boyle, J.J., Branca, J., Bedard, O., Hasham, M.G., Benson, L., Harding, S.E., Rosenthal, N., and Sattler, S., Type 2 MI induced by a single high dose of isoproterenol in C57BL/6J mice triggers a persistent adaptive immune response against the heart, J. Cell Mol. Med., 2021, vol. 25, pp. 229–243.
Frobert, A., Valentin, J., Magnin, J.L., Riedo, E., Cook, S., and Giraud, M.N., Prognostic value of troponin I for infarct size to improve preclinical myocardial infarction small animal models, Front. Physiol., 2015, vol. 6, p. 353.
Gaze, D.C., Ischemia modified albumin: a novel biomarker for the detection of cardiac ischemia, Drug Metab. Pharmacokinet., 2009, vol. 24, pp. 333–341.
Gurumurthy, P., Borra, S.K., Yeruva, R.K.R., Victor, D., Babu, S., and Cherian, K.M., Estimation of Ischemia Modified Albumin (IMA) levels in patients with acute coronary syndrome, Indian J. Clin. Biochem., 2014, vol. 29, pp. 367–371.
Hasan, R., Lasker, S., Hasan, A., Zerin, F., Zamila, M., Chowdhury, F.I., Nayan, S.I., Rahman, M.M., Khan, F., Subhan, N., and Alam, M.A., Canagliflozin attenuates isoprenaline-induced cardiac oxidative stress by stimulating multiple antioxidant and anti-inflammatory signaling pathways, Sci. Rep., 2020, vol. 10, p. 14459.
Karin, M. and Delhase, M., The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signaling, Semin. Immunol., 2000, vol. 12, pp. 85–98.
Karthikeyan, K., Bai, B.R.S., and Devaraj, S.N., Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats, Int. J. Cardiol., 2007, vol. 115, pp. 326–333.
Kono, Y., Nakamura, K., Kimura, H., Nishii, N., Watanabe, A., Banba, K., Miura, A., Nagase, S., Sakuragi, S., Kusano, K.F., Matsubara, H., and Ohe, T., Elevated levels of oxidative DNA damage in serum and myocardium of patients with heart failure, Circ. J., 2006, vol. 70, pp. 1001–1005.
Kumar, M., Kasala, E.R., Bodduluru, L.N., Dahiya, V., and Lahkar, M., Baicalein protects isoproterenol induced myocardial ischemic injury in male Wistar rats by mitigating oxidative stress and inflammation, Inflamm. Res., 2016, vol. 65, pp. 613–622.
Kusaka, H., Koibuchi, N., Hasegawa, Y., Ogawa, H., and Kim-Mitsuyama, S., Empagliflozin lessened cardiac injury and reduced visceral adipocyte hypertrophy in prediabetic rats with metabolic syndrome, Cardiovasc. Diabetol., 2016, vol. 15, p. 157.
Li, H., Xie, Y.H., Yang, Q., Wang, S.W., Zhang, B.L., Wang, J.B., Cao, W., Bi, L.L., Sun, J.Y., Miao, S., Hu, J., Zhou, X.X., and Qiu, P.C., Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats, PLoS One, 2012, vol. 7, p. e48872.
Li, J., Thangaiyan, R., Govindasamy, K., and Wei, J., Anti-inflammatory and anti-apoptotic effect of zingiberene on isoproterenol-induced myocardial infarction in experimental animals, Hum. Exp. Toxicol., 2021, vol. 40, pp. 915–927.
Lin, B., Koibuchi, N., Hasegawa, Y., Sueta, D., Toyama, K., Uekawa, K., Ma, M., Nakagawa, T., Kusaka, H., and Kim-Mitsuyama, S., Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice, Cardiovasc. Diabetol., 2014, vol. 13, p. 148.
Lobo Filho, H.G., Ferreira, N.L., Sousa, R.B. de, Carvalho, E.R. de, Lobo, P.L.D., and Lobo Filho, J.G., Experimental model of myocardial infarction induced by isoproterenol in rats. Rev. Bras. Cir. Cardiovasc., 2011, vol. 26, pp. 469–476.
Loh, H.K., Sahoo, K.C., Kishore, K., Ray, R., Nag, T.C., Kumari, S., and Arya, D.S., Effects of thalidomide on isoprenaline-induced acute myocardial injury: a haemodynamic, histopathological and ultrastructural study, Basic Clin. Pharmacol. Toxicol., 2007, vol. 100, pp. 233–239.
Maneewong, K., Mekrungruangwong, T., Luangaram, S., Thongsri, T., and Kumphune, S., Combinatorial determination of ischemia modified albumin and protein carbonyl in the diagnosis of nonST-elevation myocardial infarction, Indian J. Clin. Biochem., 2011, vol. 26, pp. 389–395.
Mizuno, M., Kuno, A., Yano, T., Miki, T., Oshima, H., Sato, T., Nakata, K., Kimura, Y., Tanno, M., and Miura, T., Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts, Physiol. Rep., 2018, vol. 6, p. e13741.
Nagoor Meeran, M.F., Stanely Mainzen Prince, P., and Hidhayath Basha, R., Preventive effects of N-acetyl cysteine on lipids, lipoproteins and myocardial infarct size in isoproterenol induced myocardial infarcted rats: an in vivo and in vitro study, Eur. J. Pharmacol., 2012, vol. 677, pp. 116–122.
Neal, B., Perkovic, V., Mahaffey, K.W., de Zeeuw, D., Fulcher, G., Erondu, N., Shaw, W., Law, G., Desai, M., and Matthews, D.R., Canagliflozin and cardiovascular and renal events in type 2 diabetes, N. Engl. J. Med., 2017, vol. 377, pp. 644–657.
Norbury, C.J. and Zhivotovsky, B., DNA damage-induced apoptosis, Oncogene, 2004, vol. 23, pp. 2797–2808.
O’Brien, P.J., Smith, D.E.C., Knechtel, T.J., Marchak, M.A., Pruimboom-Brees, I., Brees, D.J., Spratt, D.P., Archer, F.J., Butler, P., Potter, A.N., Provost, J.P., Richard, J., Snyder, P.A., and Reagan, W.J., Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals, Lab. Anim., 2006, vol. 40, pp. 153–171.
Oelze, M., Kröller-Schön, S., Welschof, P., Jansen, T., Hausding, M., Mikhed, Y., Stamm, P., Mader, M., Zinßius, E., Agdauletova, S., Gottschlich, A., Steven, S., Schulz, E., Bottari, S.P., Mayoux, E., Münzel, T., and Daiber, A., The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity, PLoS One, 2014, vol. 9, p. e112394.
Oktar, S., Aydin, M., Yönden, Z., Alçin, E., Ilhan, S., and Nacar, A., Effects of caffeic acid phenethyl ester on isoproterenol-induced myocardial infarction in rats, Anadolu Kardiyol. Derg., 2010, vol. 10, pp. 298–302.
Oshima, H., Miki, T., Kuno, A., Mizuno, M., Sato, T., Tanno, M., Yano, T., Nakata, K., Kimura, Y., Abe, K., Ohwada, W., and Miura, T., Empagliflozin, an SGLT2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats, J. Pharmacol. Exp. Ther., 2019, vol. 368, pp. 524–534.
Othman, A.I., Elkomy, M.M., El-Missiry, M.A., and Dardor, M., Epigallocatechin-3-gallate prevents cardiac apoptosis by modulating the intrinsic apoptotic pathway in isoproterenol-induced myocardial infarction, Eur. J. Pharmacol., 2017, vol. 794, pp. 27–36.
Panda, S., Kar, A., and Biswas, S., Preventive effect of agnucastoside C against isoproterenol-induced myocardial injury, Sci. Rep., 2017, vol. 7, p. 16146.
Panda, V.S. and N, S.R., Evaluation of cardioprotective activity of Ginkgo biloba and Ocimum sanctum in rodents, Altern. Med. Rev., 2009, vol. 14, pp. 161–171.
Patel, V., Upaganlawar, A., Zalawadia, R., and Balaraman, R., Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: a biochemical, electrocardiographic and histoarchitectural evaluation, Eur. J. Pharmacol., 2010, vol. 644, pp. 160–168.
Prince, P.S.M., A biochemical, electrocardiographic, electrophoretic, histopathological and in vitro study on the protective effects of (–)epicatechin in isoproterenol-induced myocardial infarcted rats, Eur. J. Pharmacol., 2011, vol. 671, pp. 95–101.
Radhiga, T., Rajamanickam, C., Sundaresan, A., Ezhumalai, M., and Pugalendi, K.V., Effect of ursolic acid treatment on apoptosis and DNA damage in isoproterenol-induced myocardial infarction, Biochimie, 2012, vol. 94, pp. 1135–1142.
Raish, M., Momordica charantia polysaccharides ameliorate oxidative stress, hyperlipidemia, inflammation, and apoptosis during myocardial infarction by inhibiting the NF-κB signaling pathway, Int. J. Biol. Macromol., 2017, vol. 97, pp. 544–551.
Rani, N., Bharti, S., Bhatia, J., Tomar, A., Nag, T.C., Ray, R., and Arya, D.S., Inhibition of TGF-β by a novel PPAR-γ agonist, chrysin, salvages β-receptor stimulated myocardial injury in rats through MAPKs-dependent mechanism, Nutr. Metab., 2015, vol. 12, p. 11.
Rona, G., Chappel, C.I., Balazs, T., and Gaudry, R., An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat, A.M.A. Arch. Pathol., 1959, vol. 67, pp. 443–455.
Savarimuthu, S. and Harky, A., The role of sodium-glucose co-transporter 2 protein inhibitors in heart failure: more than an antidiabetic drug?, Expert. Opin. Pharmacother., 2022, vol. 23, pp. 377–386.
Searle, A.J. and Willson, R.L., Glutathione peroxidase: effect of superoxide, hydroxyl and bromine free radicals on enzyme activity, Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med., 1980, vol. 37, pp. 213–217.
Sharma, H.S. and Das, D.K., Role of cytokines in myocardial ischemia and reperfusion, Mediators Inflamm., 1997, vol. 6, pp. 175–183.
Soraya, H., Khorrami, A., Garjani, Afagh, Maleki-Dizaji, N., and Garjani, A., Acute treatment with metformin improves cardiac function following isoproterenol induced myocardial infarction in rats, Pharmacol. Rep., 2012, vol. 64, pp. 1476–1484.
Tang, Y.-N., He, X.-C., Ye, M., Huang, H., Chen, H.-L., Peng, W.-L., Zhao, Z.-Z., Yi, T., and Chen, H.-B., Cardioprotective effect of total saponins from three medicinal species of Dioscorea against isoprenaline-induced myocardial ischemia, J. Ethnopharmacol., 2015, vol. 175, pp. 451–455.
Thangaiyan, R., Arjunan, S., Govindasamy, K., Khan, H.A., Alhomida, A.S., and Prasad, N.R., Galangin attenuates isoproterenol-induced inflammation and fibrosis in the cardiac tissue of albino Wistar rats, Front. Pharmacol., 2020, vol. 11, p. 585163.
Toker, A., Aribas, A., Yerlikaya, F.H., Tasyurek, E., and Akbuğa, K., Serum and saliva levels of ischemia-modified albumin in patients with acute myocardial infarction, J. Clin. Lab. Anal., 2013, vol. 27, pp. 99–104.
Tucker, J.F., Collins, R.A., Anderson, A.J., Hauser, J., Kalas, J., and Apple, F.S., Early diagnostic efficiency of cardiac troponin I and Troponin T for acute myocardial infarction. Acad. Emerg. Med., 1997, vol. 4, pp. 13–21.
Upaganlawar, A., Gandhi, H., and Balaraman, R., Effect of vitamin E alone and in combination with lycopene on biochemical and histopathological alterations in isoproterenol-induced myocardial infarction in rats, J. Pharmacol. Pharmacother., 2010, vol. 1, pp. 24–31.
Uygun, M., Yilmaz, S., Pekdemir, M., Duman, C., and Gürbüz, Y.S., The diagnostic value of ischemia-modified albumin in a rat model of acute mesenteric ischemia, Acad. Emerg. Med., 2011, vol. 18, pp. 355–359.
Verma, S., Rawat, S., Ho, K.L., Wagg, C.S., Zhang, L., Teoh, H., Dyck, J.E., Uddin, G.M., Oudit, G.Y., Mayoux, E., Lehrke, M., Marx, N., and Lopaschuk, G.D., Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors, JACC Basic Transl. Sci., 2018, vol. 3, pp. 575–587.
Wattanapitayakul, S.K. and Bauer, J.A., Oxidative pathways in cardiovascular disease: roles, mechanisms, and therapeutic implications, Pharmacol. Ther., 2001, vol. 89, pp. 187–206.
Widyaningsih, W., Pramono, S., Zulaela, Sugiyanto, and Widyarini, S., Protection by ethanolic extract from Ulva lactuca L. against acute myocardial infarction: antioxidant and antiapoptotic activities, Malays. J. Med. Sci., 2017, vol. 24, pp. 39–49.
Woudstra, L., Biesbroek, P.S., Emmens, R.W., Heymans, S., Juffermans, L.J., van Rossum, A.C., Niessen, H.W.M., and Krijnen, P.A.J., Lymphocytic myocarditis occurs with myocardial infarction and coincides with increased inflammation, hemorrhage and instability in coronary artery atherosclerotic plaques, Int. J. Cardiol., 2017, vol. 232, pp. 53–62.
Zhang, B., Wang, H., Yang, Z., Cao, M., Wang, K., Wang, G., and Zhao, Y., Protective effect of alpha-pinene against isoproterenol-induced myocardial infarction through NF-κB signaling pathway, Hum. Exp. Toxicol., 2020, vol. 39, pp. 1596–1606.
Zhang, W., Li, Y., and Ge, Z., Cardiaprotective effect of crocetin by attenuating apoptosis in isoproterenol induced myocardial infarction rat model, Biomed. Pharmacother., 2017, vol. 93, pp. 376–382.
Zinman, B., Wanner, C., Lachin, J.M., Fitchett, D., Bluhmki, E., Hantel, S., Mattheus, M., Devins, T., Johansen, O.E., Woerle, H.J., Broedl, U.C., and Inzucchi, S.E., Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes, N. Engl. J. Med., 2015, vol. 373, pp. 2117–2128.
ACKNOWLEDGMENTS
We thank Amanda Chilaka (microbiologist at the Whitehead Institute in Boston) for language editing of the manuscript.
Funding
This research was supported by Sivas Cumhuriyet University Scientific Research Projects Commission as research project no. V-092.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Mehmet Ekici, Güngör, H., Karayığıt, M.Ö. et al. Cardioprotective Effect of Empagliflozin in Rats with Isoproterenol-Induced Myocardial Infarction: Evaluation of Lipid Profile, Oxidative Stress, Inflammation, DNA Damage, and Apoptosis. Biol Bull Russ Acad Sci 49 (Suppl 1), S159–S172 (2022). https://doi.org/10.1134/S1062359022130039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359022130039