Abstract
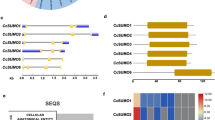
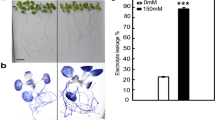
This study is aimed at investigating whether overexpression of the heat shock protein gene (AsHSP70) isolated from Agave sisalana will improve the heat stress tolerance in plants. AsHSP70 gene with a full-length cDNA of 1800 bp, encoding 282 amino acids, was functionally characterized. Bioinformatics tools showed the theoretical isoelectric point is pI = 4.780, located in the nucleus with 76.8% reliability having an N-terminal signal domain. 3D model, showed an ATP and ADP ligands with –6.2 and 6.5 kJ/mol binding affinity respectively. AsHSP70 was cloned in Plant expression vector in fusion with Green Florescent Protein from pGWB5 vector. Syringe agroinfiltration of Agrobacterium tumefaciens harboring AsHSP70/GFP was done into leaf of Nicotiana benthamiana. Spermidine was applied to the infiltrated tobacco plants for exogenous induction of polyamines under heat stress. Relative expression of AsHSP70 was significant in tobacco leaf under heat stress. Membrane stability index was about 95.59 and 96.53% and leaf relative water content was 4.43 and 3.44% respectively in infiltrated and polyamine-induced plants. Proline and sugar content were also increased under heat stress. Hence, we established that AsHSP70 is expressing transiently under heat stress in tobacco as model species and further genetic transformation of this gene will lead to induce the stress tolerance in agriculturally important crops.








Similar content being viewed by others
REFERENCES
Basaran, P. and Rodríguez-Cerezo, E., Plant molecular farming: opportunities and challenges, Critic. Rev. Biotechnol., 2008, vol. 28, pp. 153–172. https://doi.org/10.1080/07388550802046624
Batcho, A.A., Rashid, B., Hassan, S., and Husnain, T., Genetic transformation of heat shock protein gene (HSP70) family in Cotton and its expression in transgenic plants under heat stress, Plant Mol. Biol., 2020 (submitted).
Batcho, A.A., Sarwar, M.B., Tariq, L., Rashid, B., Hassan, S., and Husnain, T., Identification and characterisation of heat shock protein gene (HSP70) family and its expression in Agave sisalana under heat stress, J. Hort. Sci. Biotechnol., 2019. https://doi.org/10.1080/14620316.2019.1685412
Bates, L.S., Waldren, R.P., and Teare, I.D., Rapid determination of free proline for water stress studies, Plant Soil, 1973, vol. 39, pp. 205–207. https://doi.org/10.1007/BF00018060
Cao, S., Song, C., Shao, J., Bian, K., Chen, W., and Yang, Z., Exogenous melatonin treatment increases chilling tolerance and induces defense response in harvested peach fruit during cold storage, J. Agri. Food Chem., 2016, vol. 64, pp. 5215–5222. https://doi.org/10.1021/acs.jafc.6b01118
Chen, D., Shao, Q., Yin, L., Younis, A., and Zheng, B., Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses, Front. Plant Sci., 2019. https://doi.org/10.3389/fpls.2018.01945
Chen, N., Wang, W.-M., and Wang, H.-L., An efficient full-length cDNA amplification strategy based on bioinformatics technology and multiplexed PCR methods, Sci. Rep., 2016, vol. 6. https://doi.org/10.1038/srep19420
Chen, Q., Lai, H., Hurtado, J., Stahnke, J., Leuzinger, K., and Dent, M., Agroinfiltration as an effective and scalable strategy of gene delivery for production of pharmaceutical proteins, Adv. Tech. Biol. Med., 2013, vol. 1, pp. 103–110. https://doi.org/10.4172/atbm.1000103
Chen, W., Xie, S.S., Zeng, F., and Li, B., A new process knowledge representation approach using parameter flow chart, Comput. Indus., 2011, vol. 62, pp. 9–22. https://doi.org/10.1016/j.compind.2010.05.016
Chow, P.S. and Landhäusser, S.M., A method for routine measurements of total sugar and starch content in woody plant tissues, Tree Physiol., 2004, vol. 24, pp. 1129–1136. https://doi.org/10.1093/treephys/24.10.1129
Coleman-Derr, D., Desgarennes, D., Fonseca-Garcia, C., Gross, S., Clingenpeel, S., Woyke, T., North, G., Visel, A., Partida-Martinez, L.P., and Tringe, S.G., Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species, New Phytol., 2016, vol. 209, pp. 798–811. https://doi.org/10.1111/nph.13697
DaMatta, F.M. and Ramalho, J.D.C., Impacts of drought and temperature stress on coffee physiology and production: a review, Brazil. J. Plant Physiol., 2006, vol. 18, pp. 55–81. https://doi.org/10.1590/S1677-04202006000100006
Farooq, M., Bramley, H., Palta, J.A., and Siddique, K.H.M., Heat stress in wheat during reproductive and grain-filling phases, Critic. Rev. Plant Sci., 2011, vol. 30, pp. 491–507. https://doi.org/10.1080/07352689.2011.615687
Hasanuzzaman, M., Nahar, K., Alam, M., Roychowdhury, R., and Fujita, M., Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants, Intl. J. Mol. Sci., 2013, vol. 14, pp. 9643–9684. https://doi.org/10.3390/ijms14059643
Hupało, K., Riss, H.W., and Grabowski, M., Climate change as a possible driver of invasion and differential in HSP70 expression in two genetically distinct populations of the invasive killer shrimp, Dikerogammarus villosus, Biol. Invasions, 2018, vol. 20, pp. 2047–2059. https://doi.org/10.1007/s10530-018-1679-2
Lalitha, S., Primer premier 5, Biotech Software Internet Rep., 2000, vol. 1, pp. 270–272. https://doi.org/10.1089/152791600459894
Li, Z., Yu, J., Peng, Y., and Huang, B., Metabolic pathways regulated by abscisic acid, salicylic acid and γ aminobutyric acid in association with improved drought tolerance in creeping bentgrass (Agrostis stolonifera), Physiol. Plant., 2017, vol. 159, pp. 42–58. https://doi.org/10.1111/ppl.12483
Malabadi, R.B., Teixeira da-Silva, J.A., and Nataraja, K., Green fluorescent protein in the genetic transformation of plants, Transgen. Plant J., 2008, vol. 2, pp. 86–109.
Muoki, R.C., Paul, A., Kumari, A., Singh, K., and Kumar, S., An improved protocol for the isolation of RNA from roots of tea (Camellia sinensis (L.) O. Kuntze), Mol. Biotechnol., 2012, vol. 52, pp. 82–88. https://doi.org/10.1007/s12033-011-9476-5
Ni, Z., Li, H., Zhao, Y., Peng, H., Hu, Z., Xin, M., and Sun, Q., Genetic improvement of heat tolerance in wheat: recent progress in understanding the underlying molecular mechanisms, Crop J., 2018, vol. 6, pp. 32–41. https://doi.org/10.1016/j.cj.2017.09.005
Ogburn, R.M. and Edwards, E.J., The ecological water-use strategies of succulent plants, in Advances in Botanical Research, Kader, J.-C. and Delseny, M., Eds., Elsevier, 2010, pp. 179–225. https://doi.org/10.1016/B978-0-12-380868-4.00004-1
Reddy, A.R., Chaitanya, K.V., and Vivekanandan, M., Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants, J. Plant Physiol., 2004, vol. 161, pp. 1189–1202. https://doi.org/10.1016/j.jplph.2004.01.013
Sarwar, M.B., Ahmad, Z., Batcho, A.A., Rashid, B., Hassan, S., Ahmad, A., and Husnain, T., Identification and validation of superior housekeeping gene(s) for qRT-PCR data normalization in Agave sisalana (a CAM-plant) under abiotic stresses, Physiol. Mol. Biol. Plants., 2020, vol. 26, pp. 567–584. https://doi.org/10.1007/s12298-020-00760-y
Sarwar, M.B., Ahmad, Z., Rashid, B., Hassan, S., Gregersen, P.L., Leyva, M.dlo., Nagy, I., Asp, T., and Husnain, T., De novo assembly of Agave sisalana transcriptome in response to drought stress provides insight into the tolerance mechanisms, Sci. Rep., 2019, vol. 9, p. 396. https://doi.org/10.1038/s41598-018-35891-6
Singh, A. and Grover, A., Genetic engineering for heat tolerance in plants, Physiol. Mol. Biol. Plants., 2008, vol. 14, pp. 155–165. https://doi.org/10.1007/s12298-008-0014-2
Snow, A.A., Andow, D.A., Gepts, P., Hallerman, E.M., Power, A., Tiedje, J.M., and Wolfenbarger, L., Genetically engineered organisms and the environment: current status and recommendations, Ecol. Appl., 2005, vol. 15, pp. 377–404. https://doi.org/10.1890/04-0539
Tedeschi, J., Kennington, W., Berry, O., Whiting, S., Meekan, M., and Mitchell, N., Increased expression of Hsp70 and Hsp90 mRNA as biomarkers of thermal stress in loggerhead turtle embryos (Caretta caretta), J. Thermal Biol., 2015, vol. 47, pp. 42–50. https://doi.org/10.1016/j.jtherbio.2014.11.006
Vickers, C.E., Gershenzon, J., Lerdau, M.T., and Loreto, F., A unified mechanism of action for volatile isoprenoids in plant abiotic stress, Natl. Chem. Biol., 2009, vol. 5, p. 283. https://doi.org/10.1038/nchembio.158
ACKNOWLEDGMENTS
The authors would like to acknowledge the Higher Education Commission Pakistan (HEC) and The World Academy of Science (TWAS) for provision of funds to complete this study.
Author information
Authors and Affiliations
Contributions
This study has been designed by TH and managed by BR. The overall experiments have been done by BAA, Bioinformatics analyses has been performed by BJ, Real time qPCR has been done by SMB. Statistical and graphical data presentation was done by SH. Manuscript drafted by BR and TH edited the final version. All the authors have read and approved the manuscript.
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Batcho, A.A., Jabbar, B., Sarwar, M.B. et al. Transient Expression Analysis of Agave sisalana Heat Shock Protein Gene (AsHSP70) in Model Species (Nicotiana benthamiana) under Heat Stress. Biol Bull Russ Acad Sci 49, 160–168 (2022). https://doi.org/10.1134/S1062359022030037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359022030037