Abstract
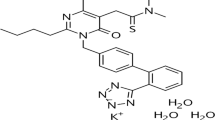
The fixed dose combination of azilsartan medoxomil potassium and chlorthalidone has been introduced for the effective treatment of hypertension. In the present work a rapid, simple and accurate stability indicating ultra HPLC assay method has been developed. The separation of azilsartan medoxomil, chlorthalidone and their degradation products were accomplished on an Acquity UPLC BEH C18 (100 mm × 2.1 mm, 1.7 μm) column using mobile phase combination of 0.02% trifluoroacetic acid in water and acetonitrile in gradient mode. The forced degradation products were identified using liquid chromatography‒electrospray ionisation-quadrupole time of flight-tandem mass spectrometry (LC‒ESIQTOF–MS/MS) and accurate mass experiments. The in silico toxicities of the degradation products for both the drugs were evaluated. The proposed method was validated as per the ICH Q2 (R1) guideline for selectivity, linearity, precision, accuracy and robustness.
Similar content being viewed by others
References
Baker, W.L. and White, W.B., Ann. Pharmacother., 2011, vol. 45, no. 12, p. 1506.
Lam, S., Cardiol. Rev., 2011, vol. 19, no. 6, p. 300.
Sica, D., White, W.B., Weber, M.A., Bakris, G.L., Perez, A., Cao, C., Handley, A., and Kupfer, S., J. Clin. Hypertens., 2011, vol. 13, no. 7, p. 467.
Ernst, M.E., Carter, B.L., Goerdt, C.J., Steffensmeier, J.J., Phillips, B.B., Zimmerman, M.B., and Bergus, G.R., Hypertension, 2006, vol. 47, no. 3, p. 352.
Cushman, W.C., Bakris, G.L., White, W.B., Weber, M.A., Sica, D., Roberts, A., Lloyd, E., and Kupfer, S., Hypertension, 2012, vol. 60, no. 2, p. 310.
Reynolds, D.W., Facchine, K.L., Mullaney, J.F., Alsante, K.M., Hatajik, T.D., and Motto, M.G., Pharm. Technol., 2002, vol. 26, p. 48.
Wegienka, L.C. and Weller, J.M., Arch. Int. Med., 1964, vol. 114, no. 2, p. 232.
Blessy, M., Patel, R.D., Prajapati, P.N., and Agrawal, Y., J. Pharm. Anal., 2014, vol. 4, no. 3, p. 159.
Sravani, P., Rubesh, S., Duganath, N., and Devanna, N., Int. J. Pharma Sci., 2014, vol. 4, no. 5, p. 725.
Swain, D., Patel, P.N., Palaniappan, I., Sahu, G., and Samanthula, G., Rapid Commun. Mass Spectrom., 2015, vol. 29, no. 15, p. 1437.
Swain, D., Sahu, G., and Samanthula, G., J. Anal. Bioanal. Tech., 2015, vol. 6, no. 4, 254. doi 10.4172/2155-9872.1000254
Q1B Stability Testing: Photostability Testing of New Drug Substances and Products, Proc. Int. Conf. Harmonization, Geneva, 1996.
Q1A(R2) Stability Testing of New Drug Substances and Products, Proc. Int. Conf. Harmonization, Geneva, 2003.
Q2 (R1) Validation of Analytical Procedures, Proc. Int. Conf. Harmonization, Geneva, 2005.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Samanthula, G., Swain, D., Sahu, G. et al. Ultra HPLC Method for Fixed Dose Combination of Azilsartan Medoxomil and Chlorthalidone: Identification and in silico Toxicity Prediction of Degradation Products. J Anal Chem 73, 560–569 (2018). https://doi.org/10.1134/S1061934818060138
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934818060138