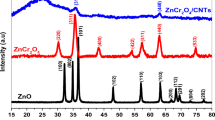
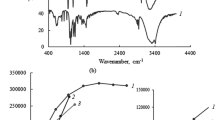
Abstract
The electrocatalytic performance of carbon paste electrode modified with ferrocene-derivative (ethyl 2-(4-ferrocenyl[1,2,3]triazol-1-yl)acetate, EFTA) , CeO–ZnO nanocomposite as well as ionic liquid (n-hexyl-3-methylimidazolium hexafluoro phosphate) (CeO–ZnO/ILFCPE) was investigated for simultaneous detection of penicillamine and tryptophan. According to the results, the penicillamine oxidation on the surface of fabricated carbon paste electrode at an optimal pH of 7.0 was observed less positive at 320 mV potential compared to unmodified electrode. The penicillamine oxidation showed the electron transfer coefficient (α) of 0.58 and diffusion coefficient (D) of 1.2 × 10–6 cm2/s. The linear dynamic range was also calculated to be between 0.02–25.0 μM, and the limit of detection (LOD) was 10.0 nM. High selectivity and satisfactory reproducibility found for the modified carbon paste electrode suggest the possibility of analytical applications. The application of the synthesized sensor was examined in real specimens for detection of penicillamine and tryptophan.










Similar content being viewed by others
REFERENCES
Li, B.L., Luo, J.H., Luo, H.Q., and Li, N.B., A novel strategy for selective determination of d-penicillamine based on molecularly imprinted polypyrrole electrode via the electrochemical oxidation with ferrocyanide, Sens. Actuators B: Chem., 2013, vol. 186, p. 96.
Li, N.B. and Kwak, J., A penicillamine biosensor based on tyrosinase immobilized on nano-Au/PAMAM dendrimer modified gold electrode, Electroanalysis, 2007, vol. 19, p. 2428.
Walshe, J.M., The story of penicillamine: a difficult birth, Mov. Disord., 2003, vol. 18, p. 853.
Wang, Q., Dong, D., and Li, N., Electrochemical response of dopamine at a penicillamine self-assembled gold electrode, Bioelectrochemistry, 2001, vol. 54, p. 169.
Felson, D.T., Anderson, J.J., and Meenan, R.F., The comparative efficacy and toxicity of second-line drugs in rheumatoid arthritis results of two metaanalyses, Arthritis Rheum., 1990, vol. 33, p. 1449.
Porifreva, A.V., Gorbatchuk, V.V., Evtugyn, V.G., Stoikov, I.I., and Evtugyn, G.A., Glassy carbon electrode modified with silver nanodendrites implemented in polylactide-thiacalix [4] arene copolymer for the electrochemical determination of tryptophan, Electroanalysis, 2018, vol. 30, p. 641.
Li, J., Kuang, D., Feng, Y., Zhang, F., Xu, Z., Liu, M., and Wang, D., Green synthesis of silver nanoparticles-graphene oxide nanocomposite and its application in electrochemical sensing of tryptophan, Biosens. Bioelectron., 2013, vol. 42, p. 198.
Mattioli, I.A., Baccarin, M., Cervini, P., and Cavalheiro, É.T., Electrochemical investigation of a graphite-polyurethane composite electrode modified with electrodeposited gold nanoparticles in the voltammetric determination of tryptophan, J. Electroanal. Chem., 2019, vol. 835, p. 212.
Idili, A., Gerson, J., Parolo, C., Kippin, T., and Plaxco, K.W., An electrochemical aptamer-based sensor for the rapid and convenient measurement of l-tryptophan, Anal. Bioanal. Chem., 2019, vol. 411, p. 2019.
Beitollahi, H., Garkani-Nejad, F., Tajik, S., and Ganjali, M.R., Voltammetric determination of acetaminophen and tryptophan using a graphite screen printed electrode modified with functionalized graphene oxide nanosheets within a Fe3O4@SiO2 nanocomposite, Iran. J. Pharm. Res., 2019, vol. 18, p. 80.
Han, J., Zhao, J., Li, Z., Zhang, H., Yan, Y., Cao, D., and Wang, G., Nanoporous carbon derived from dandelion pappus as an enhanced electrode material with low cost for amperometric detection of tryptophan, J. Electroanal. Chem., 2018, vol. 818, p. 149.
Fiorucci, A.R. and Cavalheiro, É.T.G., The use of carbon paste electrode in the direct voltammetric determination of tryptophan in pharmaceutical formulations, J. Pharm. Biomed. Anal., 2002, vol. 28, p. 909.
Gibbs, D.A. and Watts, R.W., Studies on the effect of D-penicillamine and N-acetyl-D-penicillamine on the excretion of some tryptophan metabolites in patients with cystinuria, Clin. Sci., 1969, vol. 36, p. 87.
Salmanipour, A., Taher, M.A., Beitollahi, H., and Hosseinzadeh, R., An electrochemical sensor based on 1-benzyl-4-ferrocenyl-1H-[1,2,3]-triazole/carbon nanotube; detection of D-penicillamine in the presence of tryptophan, Mater. Sci. Eng. C, 2013, vol. 33, p. 3160.
Mirrahimi, F., Taher, M.A., Beitollahi, H., and Hosseinzadeh, R., Electrocatalytic and selective determination of d-penicillamine in the presence of tryptophan using a benzoylferrocene-modified carbon nanotube paste electrode, Appl. Organomet. Chem., 2012, vol. 26, p. 194.
Biffar, S., Greely, V., and Tibbetts, D., Determination of penicillamine in encapsulated formulations by high-performance liquid chromatography, J. Chromatogr. A, 1985, vol. 318, p. 404.
Saetre, R. and Rabenstein, D.L., Determination of penicillamine in blood and urine by high performance liquid chromatography, Anal. Chem., 1978, vol. 50, p. 276.
Yamashita, G.T. and Rabenstein, D.L., Determination of penicillamine, penicillamine disulfide and penicillamine-glutathione mixed disulfide by high-performance liquid chromatography with electrochemical detection, J. Chromatogr. B: Biomed. Sci. Appl., 1989, vol. 491, p. 341.
Cavrini, V., Gatti, R., Roveri, P., and Cesaroni, M.R., Use of 4-(6-methylnaphthalen-2-yl)-4-oxobut-2-enoic acid as a reagent for the spectrophotometric and fluorimetric determination of aliphatic thiol drugs, Analyst, 1988, vol. 113, p. 1447.
Besada, A., Tadros, N.B., and Gawargious, Y.A., Spectrophotometric determination of pure and capsule-formulated Penicillamine, Anal. Lett., 1987, vol. 20, p. 809.
Besada, A., A new simple and sensitive spectrophotometric method for determination of penicillamine by reaction with nitrite and Co(II) ions, Anal. Lett., 1988, vol. 21, p. 435.
Al-Majed, A.A., Spectrophotometric estimation of d‑penicillamine in bulk and dosage forms using 2,6-dichloroquinone-4-chlorimide (DCQ), J. Pharm. Biomed. Anal., 1999, vol. 21, p. 827.
Suliman, F.E.O., Al-Lawati, H.A., Al-Kindy, S.M., Nour, I.E.M., and Salama, S.B., A sequential injection spectrophotometric method for the determination of penicillamine in pharmaceutical products by complexation with iron(III) in acidic media, Talanta, 2003, vol. 61, p. 221.
Vinas, P., Garcia, I.L., and Gil, J.M., Determination of thiol-containing drugs by chemiluminescence-flow injection analysis, J. Pharm. Biomed. Anal., 1993, vol. 11, p. 15.
Zhang, Z.D., Baeyens, W.R.G., Zhang, X.R., and Van Der Weken, G., Chemiluminescence determination of penicillamine via flow injection applying a Quinine-cerium(IV) system, Analyst, 1996, vol. 121, p. 1569.
Beitollahi, H., Safaei, M., and Tajik, S., Different electrochemical sensors for determination of dopamine as neurotransmitter in mixed and clinical samples: a review, Anal. Bioanal. Chem. Res., 2019, vol. 6, p. 81.
Karimi-Maleh, H., Karimi, F., Alizadeh, M., and Sanati, A.L., Electrochemical sensors, a bright future in the fabrication of portable kits in analytical systems, Chem. Rec., 2020, vol. 20, p. 682.
Tajik, S. and Beitollahi, H., A sensitive chlorpromazine voltammetric sensor based on graphene oxide modified glassy carbon electrode, Anal. Bioanal. Chem. Res., 2019, vol. 6, p. 171.
Tajik, S., Beitollahi, H., Garkani-Nejad, F., Kirlikovali, K.O., Van Le, Q., Jang, H.W., Varma, R.S., Farha, O.K., and Shokouhimehr, M., Recent electrochemical applications of metal-organic framework-based materials, Cryst. Growth Des., 2020, vol. 20, p. 7034.
Karimi-Maleh, H., Karimi, F., Orooji, Y., Mansouri, G., Razmjou, A., Aygun, A., and Sen, F., A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid; a highly sensitive approach for determination of cysteamine in the presence of serotonin, Sci. Rep., 2020, vol. 10, p. 11699.
Foroughi, M.M., Beitollahi, H., Tajik, S., Akbari, A., and Hosseinzadeh, R., Electrochemical determination of N-acetylcysteine and folic acid in pharmaceutical and biological samples using a modified carbon nanotube paste electrode, Int. J. Electrochem. Sci., 2014, vol. 9, p. 8407.
Karuppiah, C., Sakthinathan, S., Chen, S.M., Manibalan, K., Chen, S.M., and Huang, S.T., A non-covalent functionalization of copper tetraphenylporphyrin/chemically reduced graphene oxide nanocomposite for the selective determination of dopamine, Appl. Organomet. Chem., 2016, vol. 30, p. 40.
Beitollahi, H., Mahmoudi Moghaddam, H., and Tajik, S., Voltammetric determination of bisphenol a in water and juice using a lanthanum(III)-doped cobalt(II, III) nanocube modified carbon screen-printed electrode, Anal. Lett., 2019, vol. 52, p. 1432.
Karimi-Maleh, H., Karimi, F., Malekmohammadi, S., Zakariae, N., Esmaeili, R., Rostamnia, S., Yola, M.L., Atar, N., Movagharnezhad, S., Rajendran, S., Razmjou, A., Orooji, Y., Agarwal, S., Gupta, V.K., and Razmjou, A., An amplified voltammetric sensor based on platinum nanoparticle/polyoxometalate/two-dimensional hexagonal boron nitride nanosheets composite and ionic liquid for determination of N-hydroxysuccinimide in water samples, J. Mol. Liq., 2020, vol. 310, p. 113185.
Wangfuengkanagul, N. and Chailapakul, O., Electrochemical analysis of D-penicillamine using a boron-doped diamond thin film electrode applied to flow injection system, Talanta, 2002, vol. 58, p. 1213.
Karimi-Maleh, H., Alizadeh, M., Orooji, Y., Karimi, F., Baghayeri, M., Rouhi, J., Tajik, H., Beitollahi, S., Agarwal, V. K., Gupta, S., Rajendran, S., Rostamnia, L., Fu, F., Saberi-Movahed, S., and Malekmohammadi, S., Guanine-based DNA biosensor amplified with Pt/SWCNTs nanocomposite as analytical tool for nanomolar determination of daunorubicin as an anticancer drug: a docking/experimental investigation, Ind. Eng. Chem. Res., 2021, vol. 60, p. 816.
Tajik, S., Beitollahi, H., Garkani-Nejad, F., Safaei, M., Zhang, K., Van Le, Q., Varma, R.S., Jang, H.W., and Shokouhimehr, M., Developments and applications of nanomaterial-based carbon paste electrodes, RSC Adv., 2020, vol. 10, p. 21561.
Karimi-Maleh, H., Karimi, F., Rezapour, M., Bijad, M., Farsi, M., Beheshti, A., and Shahidi, S.A., Carbon paste modified electrode as powerful sensor approach determination of food contaminants, drug ingredients, and environmental pollutants: a review, Curr. Anal. Chem., 2019, vol. 15, p. 410.
Tajik, S., Beitollahi, H., and Biparva, P., Methyldopa electrochemical sensor based on a glassy carbon electrode modified with Cu/TiO2 nanocomposite, J. Serb. Chem. Soc., 2018, vol. 83, p. 863.
Karimi-Maleh, H., Cellat, K., Arıkan, K., Savk, A., Karimi, F., and Şen, F., Palladium-nickel nanoparticles decorated on functionalized-MWCNT for high precision non-enzymatic glucose sensing, Mater. Chem. Phys., 2020, vol. 250, p. 123042.
Tajik, S., Mahmoudi-Moghaddam, H., and Beitollahi, H., Screen-printed electrode modified with La3+-doped Co3O4 nanocubes for electrochemical determination of hydroxylamine, J. Electrochem. Soc., 2019, vol. 166, p. B402.
Miraki, M., Karimi-Maleh, H., Taher, M.A., Cheraghi, S., Karimi, F., Agarwal, S., and Gupta, V.K., Voltammetric amplified platform based on ionic liquid/NiO nanocomposite for determination of benserazide and levodopa, J. Mol. Liq., 2019, vol. 278, p. 672.
Ganjali, M.R., Salimi, H., Tajik, S., Beitollahi, H., Rezapour, M., and Larijani, B., Application of Fe3O4@SiO2/MWCNT film on glassy carbon electrode for the sensitive electroanalysis of levodopa, Int. J. Electrochem. Sci., 2017, vol. 12, no. 6, p. 5243.
Esfandiari-Baghbamidi, S., Beitollahi, H., Tajik, S., and Hosseinzadeh, R., Voltammetric sensor based on 1-benzyl-4-ferrocenyl-1H-[1,2,3]-triazole/carbon nanotube modified glassy carbon electrode; detection of hydrochlorothiazide in the presence of propranolol, Int. J. Electrochem. Sci., 2016, vol. 11, p. 10874.
Karimi-Maleh, H., Sheikhshoaie, M., Sheikhshoaie, I., Ranjbar, M., Alizadeh, J., Maxakato, N.W., and Abbaspourrad, A., A novel electrochemical epinine sensor using amplified CuO nanoparticles and an-hexyl-3-methylimidazolium hexafluorophosphate electrode, New J. Chem., 2019, vol. 43, p. 2362.
Tahernejad-Javazmi, F., Shabani-Nooshabadi, M., and Karimi-Maleh, H., Analysis of glutathione in the presence of acetaminophen and tyrosine via an amplified electrode with MgO/SWCNTs as a sensor in the hemolyzed erythrocyte, Talanta, 2018, vol. 176, p. 208.
Alavi-Tabari, S.A.R., Khalilzadeh, M.A., and Karimi-Maleh, H., Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle, J. Electroanal. Chem., 2018, vol. 811, p. 84.
Beitollahi, H., Tajik, S., Garkani-Nejad, F., and Safaei, M., Recent advances in ZnO nanostruture based electrochemical sensors and biosensors, J. Mater. Chem. B, 2020, vol. 8, p. 5826.
Khan, S.B., Faisal, M., Rahman, M.M., and Jamal, A., Exploration of CeO2 nanoparticles as a chemi-sensor and photo-catalyst for environmental applications, Sci. total Environ., 2011, vol. 409, p. 2987.
Wei, Y., Li, M., Jiao, S., Huang, Q., Wang, G., and Fang, B., Fabrication of CeO2 nanoparticles modified glassy carbon electrode and its application for electrochemical determination of UA and AA simultaneously, Electrochim. Acta, 2006, vol. 52, p. 766.
Davoudi, S., Givianrad, M.H., Saber-Tehrani, M., and Aberoomand Azar, P., A novel electrochemical sensor based on Co3O4–CeO2–ZnO multi metal oxide nanocomposite for simultaneous detection of nanomolar Pb2+ and Hg2+ in different kind of spices, Indian J. Chem. A, 2020, vol. 58, p. 1075.
Li, X., Zhao, R., Jiang, H., Zhai, Y., and Ma, P., Preparation and catalytic properties of ZnO–CeO2–TiO2 composite, Synth. React. Inorg. Met. Org. Nano-Met. Chem., 2016, vol. 46, p. 775.
Qiu, J.D., Huang, J., and Liang, R.P., Nanocomposite film based on graphene oxide for high performance flexible glucose biosensor, Sens. Actuators B: Chem., 2011, vol. 160, p. 287.
Karthick, N.A., Thangappan, R., Arivanandhan, M., Gnanamani, A., and Jayavel, R., A facile synthesis of ferrocene functionalized graphene oxide nanocomposite for electrochemical sensing of lead, J. Inorg. Organomet. Poly. Mater., 2018, vol. 28, p. 1021.
Li, G., Zeng, J., Zhao, L., Wang, Z., Dong, C., Liang, J., Zhou, Z., and Huang, Y., Amperometric cholesterol biosensor based on reduction graphene oxide-chitosan-ferrocene/platinum nanoparticles modified screen-printed electrode, J. Nanopart. Res., 2019, vol. 21, p. 162.
Anderson, J.L., Armstrong, D.W., and Wei, G.T., Ionic liquids in analytical chemistry, Anal. Chem., 2006, vol. 78, p. 2892.
Baghizadeh, A., Karimi-Maleh, H., Khoshnama, Z., Hassankhani, A., and Abbasghorbani, M., A voltammetric sensor for simultaneous determination of vitamin C and vitamin B6 in food samples using ZrO2 nanoparticle/ionic liquids carbon paste electrode, Food Anal. Methods, 2015, vol. 8, p. 549.
Mahmoudi-Moghaddam, H., Tajik, S., and Beitollahi, H., A new electrochemical DNA biosensor based on modified carbon paste electrode using graphene quantum dots and ionic liquid for determination of topotecan, Microchem. J., 2019, vol. 150, p. 104085.
Bijad, M., Karimi-Maleh, H., and Khalilzadeh, M.A., Application of ZnO/CNTs nanocomposite ionic liquid paste electrode as a sensitive voltammetric sensor for determination of ascorbic acid in food samples, Food Anal. Methods, 2013, vol. 6, p. 1639.
Beitollahi, H., Movlaee, K., Ganjali, M.R., Norouzi, P., and Hosseinzadeh, R., Application of a nanostructured sensor based on graphene-and ethyl 2-(4-ferrocenyl [1,2,3]triazol-1-yl) acetate-modified carbon paste electrode for determination of methyldopa in the presence of phenylephrine and guaifenesin, Appl. Organomet. Chem., 2018, vol. 32, p. e4243.
Bard, A.J. and Faulkner, L.R., Electrochemical Methods: Fundamentals and Applications, 2nd ed., New York: Wiley, 2001.
Galus, Z., Fundamentals of Electrochemical Analysis, New York: Ellis Horwood, 1976.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Parisa Baghbanpoor, Shishehbore, M.R., Beitollahi, H. et al. The Application of Ferrocene Derivative and CeO–ZnO Nanocomposite-Modified Carbon Paste Electrode for Simultaneous Detection of Penicillamine and Tryptophan. Russ J Electrochem 58, 235–247 (2022). https://doi.org/10.1134/S1023193522040048
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193522040048