Abstract
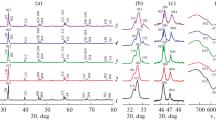
The work is focused on the studying of structural peculiarities, electronic and ionic conductivity, and thermomechanical properties of perovskite-like compositions in Ba1 –xSrxFe1 –yTiyO3 – δ and BaTi0.5Fe0.5 –zCezO3 – δ systems. The cubic structure was shown to be preserved on the substituting of up to 50% of barium cations for strontium in A-sublattice of (Ва,Sr)(Fe,Ti)O3 – δ, while further doping leads to transition of the crystal lattice into its hexagonal modification. The introducing of Ce into the B-sublattice suppressed this transformation to some extent. Substitution of titanium or cerium for iron reduced both electronic and ionic conductivity, due to the lowering of concentration of the sites available for electron transfer in the B-sublattice, lower oxygen nonstoichiometry, and larger Ti–O and Ce–O bond energy, as compared to that for Fe–O. Generally, the stabilization of the cubic structure ensures larger mobility of electronic and especially ionic charge carriers. The increasing of Ba content in the (Ва,Sr)(Fe,Ti)O3 – δ perovskite with cubic structure improved its ionic conductivity and resulted in an elongation of Fe–O bond and decreasing of the degree of overlapping between iron and oxygen atoms, which leads to lower electronic conductivity. The thermal expansion coefficients correlate with the ionic conductivity; the minimum expansibility was observed for the Ba-enriched compositions with hexagonal structure. It was demonstrated that the oxygen permeability of the (Ва,Sr)(Fe,Ti)O3 – δ and Ва(Fe,Ti,Се)O3 – δ dense membranes is limited by the oxygen diffusion in the membrane phase bulk and the oxygen surface-exchange kinetics.






Similar content being viewed by others
REFERENCES
Teraoka, Y., Zhang, H.M., Furukawa, S., and Yamazoe, N., Oxygen permeation through perovskite-type oxides, Chem. Lett., 1985, vol. 14, p. 1743.
Petric, A., Huang, P., and Tietz, F., Evaluation of La–Sr–Co–Fe–O perovskites for solid oxide fuel cells and gas separation membranes, Solid State Ionics, 2000, vol. 135, p. 719.
Vente, J.F., Haije, W.G., and Rak, Z.S., Performance of functional perovskite membranes for oxygen production, J. Membr. Sci., 2006, vol. 276, p. 178.
Stevenson, J.W., Armstrong, T.R., Carneim, R.D., Pederson, L.R., and Weber, W.J., Electrochemical properties of mixed conducting perovskites La1 –xMxCo1 –yFeyO3 – δ (M = Sr, Ba, Ca), J. Electrochem. Soc., 1996, vol. 143, p. 2722.
Shao, Z., Xiong, G, Tong, J., Dong, H., and Yang, W., Ba effect in doped Sr(Co0.8Fe0.2)O3 – δ on the phase structure and oxygen permeation properties of the dense ceramic membranes, Sep. Purif. Technol., 2001, vol. 25, p. 419.
Švarcová, S., Wiik, K., Tolchard, J., Bouwmeester, H.J.M., and Grande, T., Structural instability of cubic perovskite BaxSr1 –xCo1 –yFeyO3 – δ, Solid State Ionics, 2008, vol. 178, p. 1787.
Ovenstone, J., Jung, J.I., White, J.S., Edwards, D.D., and Misture, S.T., Phase stability of BSCF in low oxygen partial pressures, J. Solid State Chem., 2008, vol. 181, p. 576.
Arnold, M., Gesing, T.M., Martynczuk, J., and Feldhoff, A., Correlation of the formation and the decomposition process of the BSCF perovskite at intermediate temperatures, Chem. Mater., 2008, vol. 20, p. 5851.
Yan, A., Liu, B., Dong, Y., Tian, Z., Wang, D., and Cheng, M., A temperature programmed desorption investigation on the interaction of Ba0.5Sr0.5Co0.8Fe0.2O3 – δ perovskite oxides with CO2 in the absence and presence of H2O and O2, Appl. Catal. B, 2008, vol. 80, p. 24.
Kharton, V.V., Figueiredo, F.M., Kovalevsky, A.V., Viskup, A.P., Naumovich, E.N., Jurado, J.R., and Frade, J.R., Oxygen diffusion and thermal expansion of SrTiO3 – δ- and CaTiO3 – δ-based materials, Defect. Diffus. Forum, 2000, vol. 186, p. 119.
Steinsvik, S., Bugge, R., Glonnes, J., Tafto, J., and Norby, T., The defect structure of SrTi1 –xFexO3 –y (x = 0–0.8) investigated by electrical conductivity measurements and electron energy loss spectoscopy (EELS), J. Phys. Chem. Solids, 1997, vol. 58, p. 969.
Park, C.Y. and Jacobson, A.J., Electrical conductivity and oxygen nonstoichiometry of La0.2Sr0.8Fe0.55Ti0.45O3 – δ, J. Electrochem. Soc., 2005, vol. 152, p. J65.
Kharton, V.V., Shaula, A.L., Viskup, A.P., Avdeev, M., Yaremchenko, A.A., Patrakeev, M.V., Kurbakov, A.I., Naumovich, E.N., and Marques, F.M.B., Perovskite-like system (Sr,La)(Fe,Ga)O3 – δ: structure and ionic transport under oxidizing conditions, Solid State Ionics, 2002, vol. 150, p. 229.
Shannon, R.D., Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta. Crystal., 1976, vol. A32, p. 751.
Fagg, D.P., Kharton, V.V., Kovalevsky, A.V., Viskup, A.P., Naumovich, E.N., and Frade, J.R., The stability and mixed conductivity in La and Fe doped SrTiO3 in the search for potential SOFC anode materials, J. Eur. Ceram. Soc., 2001, vol. 21, p. 1831.
Kharton, V.V., Viskup, A.P., Kovalevsky, A.V., Figueiredo, F.M., Jurado, J.R., Yaremchenko, A.A., Naumovich, E.N., and Frade, J.R., Surface-limited ionic transport in perovskites Sr0.97(Ti,Fe,Mg)O3 – δ, J. Mater. Chem., 2001, vol. 10, p. 1161.
Gomez, M.I., Lucotti, G., de Moran, J.A., Aymonino, P.J., Pagola, S., Stephens, P., and Carbonio, R.E., Ab initio Structure Solution of BaFeO2.8 – δ, a New Polytype in the System BaFeOy (2.5 ≤ y ≤ 3.0) Prepared from the Oxidative Thermal Decomposition of BaFe[(CN)5NO] · 3H2O, J. Solid State Chem., 2001, vol. 160, p. 17.
Hayashi, H., Inaba, H., Matsuyama, M., Lan, N.G., Dokiya, M., and Takawa, H., Structural consideration on the ionic conductivity of perovskite-type oxides, Solid State Ionics, 1999, vol. 122, p. 10.
Mogensen, M., Lybye, D., Bonanos, N., Hendriksen, P.V., and Poulsen, F.W., Factors controlling the oxide ion conductivity of fluorite and perovskite structured oxides, Solid State Ionics, 2004, vol. 174, p. 279.
Zhu, X., Cong, Y., and Yang, W., Oxygen permeability and structural stability of BaCe0.15Fe0.85O3 – δ membranes, J. Membr. Sci., 2006, vol. 283, p. 38.
Marozau, I.P., Kharton, V.V., Viskup, A.P., Frade, J.R., and Samakhval, V.V., Electronic conductivity, oxygen permeability and thermal expansion of Sr0.7Ce0.3Mn1 –xAlxO3 – δ, J. Eur. Ceram. Soc., 2006, vol. 26, p. 1371.
Kolotygin, V.A., Tsipis, E.V., Shaula, A.L., Naumovich, E.N., Frade, J.R., Bredikhin, S.I., and Kharton, V.V., Transport, thermomechanical, and electrode properties of perovskite-type (La0.75 –xSr0.25 +x)0.95Mn0.5Cr0.5 –xTixO3 – δ, J. Solid State Electrochem., 2011, vol. 15, p. 313.
Funding
This work was supported by the Russian Science Foundation (project no. 17-79-30071). Experimental benches and procedures for electrical measurements were developed according to the State task of the Institute of Solid State Physics, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
Published on the basis of materials of the 5th All-Russia Conference “Fuel Cells and Power Plants Based on Them” (with international participation), Suzdal, 2018.
Rights and permissions
About this article
Cite this article
Kolotygin, V.A., Viskup, A.P., Pivak, E.V. et al. The Mixed Electronic and Ionic Conductivity of Perovskite-Like Ba1 –xSrxFe1 –yTiyO3 – δ and BaTi0.5Fe0.5 –zCezO3 – δ Solid Solutions. Russ J Electrochem 56, 110–117 (2020). https://doi.org/10.1134/S1023193520020068
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193520020068