Abstract
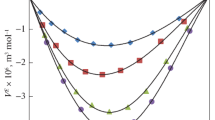
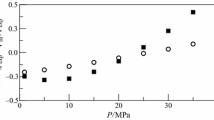
Densities and viscosities of binary mixtures of diisopropyl ether (DIPE) with methyl benzoate (MB), ethyl benzoate (EB) and benzyl acetate (BA) were measured experimentally at T = (298.15, 303.15, 308.15, and 313.15) K and P = 0.1 MPa for the entire composition range. The estimated values of the pure liquids and mixtures were subsequently used to determine the properties such as excess molar volume (VE), deviation in viscosity (∆η), and excess Gibbs free energy (∆G*E). These results were fitted into Redlich–Kister polynomial equation to calculate the coefficients and standard errors.






Similar content being viewed by others
Abbreviations
- ρ:
-
density (g/cm3);
- η:
-
dynamic viscosity (mPa s);
- V E :
-
excess molar volume (cm3/mol);
- ∆η:
-
viscosity deviation (mPa s);
- ∆G*E :
-
excess Gibbs free energy of activation flow (J/mol).
REFERENCES
J. Soujanya, B. Satyavathi, and T. Sankarshana, Fluid Phase Equilib. 382, 286 (2014).
N. Munoz-Rujas, J. P. Bazile, F. Aguilar, G. Galliero, E. Montero, and J. L. Daridon, Fluid Phase Equilib. 449, 148 (2017).
G. Sankara Reddy, A. Subba Reddy, M. Venkata Subbaiah, K. Vasudha, and A. Krishnaiah, J. Ind. Eng. Chem. 16, 941 (2010).
K. Saravanakumar, T. G. Lavanya, R. Baskaran, and T. R. Kubendran, Russ. J. Phys. Chem. A 86, 647 (2012).
D. Venkatesan and D. Joshua Amarnath, Rasayan. J. Chem. 12, 589 (2019).
D. Venkatesan, D. Joshua Amarnath, E. Sinduri, and K. Saravanakumar, Russ. J. Phys. Chem. A 93, 1489 (2012).
D. Venkatesan and D. Joshua Amarnath, Rasayan. J. Chem. 11, 834 (2018).
T. M. Aminabhavi, H. T. S. Phayde, R. S. Khinnavar, B. Gopalakrishna, and K. C. Hansen, J. Chem. Eng. Data 39, 251 (1994).
Yaw-Wen Sheu and Chein-Hsiun Tu, J. Chem. Eng. Data 50, 1706 (2005).
Hui-Wen Chen and Chein-Hsiun Tu, J. Chem. Eng. Data 51, 261 (2006).
Xianyang Meng, Jiangtao Wu, and Zhigang Liu, J. Chem. Eng. Data 54, 2353 (2009).
D. F. Montano, S. Martın, P. Cea, M. C. Lopez, and H. Artigas, J. Chem. Eng. Data 55, 5953 (2010)
T. M. Aminabhavi, Sh. K. Raikar, and R. H. Balundgi, J. Chem. Eng. Data 38, 441 (1993).
Manapragada, V. Rathnam, Sh. Mankumare, and M. S. S. Kumar, J. Chem. Eng. Data 55, 1354 (2010).
Yaw-Wen Sheu and Chein-Hsiun Tu, J. Chem. Eng. Data 51, 545 (2006).
Hyeon-DeokKim, In-Chan Hwang, and So-Jin Park, J. Chem. Eng. Data 54, 3051 (2009).
Manapragada V. Rathnam, Sh. Mankumare, K. Jain, and M. S. S. Kumar, J. Solution Chem. 41, 475 (2012).
Kai-Di Chen, Yi-Feng Lin, and Chein-Hsiun Tu, J. Chem. Eng. Data 57, 1118 (2012).
Manapragada V. Rathnam, R. K. R. Singh, and M. S. Kumar, J. Chem. Eng. Data 53, 265 (2008).
O. Redlich and A. T. Kister, Ind. Eng. Chem. 40, 345 (1948).
ACKNOWLEDGMENTS
I express my sincere gratitude to the management of Sathyabama Institute of Science and Technology for availing research facilities to carry out and publish this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
There is no conflict of interest.
Rights and permissions
About this article
Cite this article
Venkatesan, D., Amarnath, D.J. & Saravanakumar, K. Thermophysical Properties of Binary Mixtures of Diisopropyl Ether with Methyl Benzoate, Ethyl Benzoate, and Benzyl Acetate at Various Temperatures. Russ. J. Phys. Chem. 94, 1573–1580 (2020). https://doi.org/10.1134/S0036024420080300
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420080300