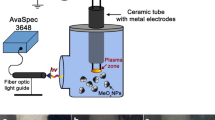
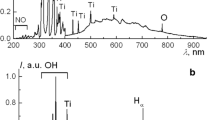
Abstract
The synthetic method used to manufacture nanostructures affects the properties of the product material. Traditional preparative methods require further purification of the product compounds from unreacted precursors and synthesis by-products and their recycling. The combination of low-temperature plasma generated between two metal electrodes and distilled water avoids those disadvantages. Here, the experience of using in-solution burning plasma in the synthesis of nanostructured inorganic materials is generalized. The structures manufactured with electrodes made of one or two materials have been studied. The thus manufactured nanostructures were characterized using scanning electron microscopy and X-ray powder diffraction. It has been found that oxide nanostructures with metals in different oxidation states can be manufactured under the underwater plasma conditions. An option to produce metal–polymer nanocomposites, doped oxide nanostructures, mixed oxides, and nanoalloys has been shown. A formation mechanism of metal oxides in the plasma zone has been suggested. The results of using the manufactured nanomaterials as photocells, sorbents of organic and inorganic contaminants, and bactericidal agents are presented.







Similar content being viewed by others
REFERENCES
I. Adamovich, S. D. Baalrud, A. Bogaerts, et al., J. Phys. D: Appl. Phys. 50, 323001 (2017).
C. Hou, M. Zhang, T. Kasama, et al., Adv. Mater. 28, 4097 (2016).
N. A. Bulychev, M. A. Kazaryan, L. L. Chaikov, et al., Bull. Lebedev Phys. Inst. 41, 264 (2014).
S. C. Singh, R. K. Swarnkar, and R. Gopal, J. Nanosci. Nanotechnol. 9, 5367 (2009).
T. Sasaki, Y. Shimizu, and N. Koshizaki, J. Photochem. Photobiol. A 182, 335 (2006).
A. A. Ashkarran, J. Cluster. Sci. 22, 233 (2011).
A. V. Khlyustova, N. A. Sirotkin, A. S. Krayev, et al., Plasma Sci. Technol. 21, 025505 (2019).
N. A. Sirotkin, A. V. Khlyustova, V. A. Titov, et al., Plasma Chem. Plasma Process. 40, 571 (2020).
K. Petcharoen and A. Sirivat, Mater. Sci. Eng. 177, 421 (2012).
A. J. Ahamed and P. V. Kumar, J. Chem. Pharm. Res. 8, 624 (2016).
E. Cherian, A. Rajan, and G. Baskar, Int. J. Modern Sci. Technol. 1, 17 (2016).
A. N. P. Madathil, K. A. Vanaja, and M. K. Jayaraj, Nanophotonic Mater. IV 6639, 66390J (2007).
M. Panahi-Kalamuei, S. Alizade, M. Mousavi-Kamazani, et al., J. Ind. Eng. Chem. 21, 1301 (2015).
A. Yan, X. Liu, G. Qiu, et al., J. Alloys Compd. 458, 487 (2008).
D. Mishra, R. Arora, S. Lahiri, et al., Prot. Met. Phys. Chem. Surf. 50, 628 (2014).
R. M. Alwan, Q. A. Kadhim, K. M. Sahan, et al., Nanosci. Nanotechnol. 5, 1 (2015).
M. Alagiri, S. Ponnusamy, and C. Muthamizhchelvan, J. Mater. Sci.: Mater. Electron 23, 728 (2012).
M. Parashar, V. K. Shukla, and R. Singh, J. Mater. Sci.: Mater. Electron. 31, 3729 (2020).
D. A. Shutov, V. V. Rybkin, A. N. Ivanov, and K. V. Smirnova, High Energy Chem. 51, 65 (2017).
T. A. Kareem and A. A. Kaliani, Ionics 18, 315 (2012).
A. Allagui, E. A. Baranova, and R. Wuthrich, Electrochim. Acta 93, 137 (2013).
N. Shirai, S. Uchida, and F. Tochikubo, Jpn. J. Appl. Phys. 53, 046202 (2014).
G. Saito and T. Akiyama, J. Nanomater. 16, 299 (2015).
D. A. Shutov, A. N. Ivanov, A. V. Rakovskaya, et al., J. Phys. D: Appl. Phys. 53, 445202 (2020).
D. A. Shutov, K. V. Smirnova, M. V. Gromov, et al., Plasma Chem. Plasma Process. 38, 107 (2018).
V. I. Yukhvid, Self-Propagating High-Temperature Synthesis: Theory and Practice (Territoriya, Chernogolovka, 2001) [in Russian].
A. Khlyustova, N. Sirotkin, A. Kraev, et al., Materialia 16, 101081 (2021).
A. Khlyustova, N. Sirotkin, V. Titov, and A. Agafonov, Curr. Appl. Phys. 20, 1396 (2020).
A. V. Khlyustova, N. A. Sirotkin, A. S. Kraev, et al., Plasma Chem. Plasma Process. 41, 643 (2021).
C. N. R. Rao, K. Biswas, K. S. Subrahmanyam, and A. Govindaraj, J. Mater. Chem. 19, 2457 (2009).
B. K. Saikia, R. K. Boruah, and P. K. Gogoi, J. Chem. Sci. 121, 103 (2009).
A. Khlyustova, N. Sirotkin, V. Titov, and A. Agafonov, J. Alloys Compd. 858, 157664 (2021).
X. Yu, T. J. Marks, and A. Facchetti, Nature Mater. 15, 383 (2016).
W. Wu, M. Wang, J. Ma, et al., Adv. Electron. Mater. 4, 1800185 (2018).
Z. M. Dang, J. K. Yuan, S. H. Yao, and R. J. Liao, Adv. Mater. 25, 6334 (2013).
G. A. Salvatore, N. Munzenrieder, T. Kinkeldei, et al., Nature Commun. 5, 1 (2014).
N. A. Sirotkin, D. L. Gurina, A. V. Khlyustova, et al., Plasma Process. Polym. 18, 2000169 (2021).
A. Khlyustova, N. Sirotkin, A. Kraev, et al., J. Appl. Polym. Sci. 138, 51174 (2021).
V. Titov, D. Nikitin, I. Naumova, et al., Materials 13, 4821 (2020).
A. Khlyustova, N. Sirotkin, T. Kusova, et al., Mater. Adv. 1, 1193 (2020).
A. Khlyustova, N. Sirotkin, A. Kraev, et al., J. Chem. Technol. Biotechnol. 96, 1125 (2021).
A. Khlyustova, N. Sirotkin, A. Kraev, et al., Dalton Trans. 49, 6270 (2020).
Funding
This work was fulfilled in the frame of the State Assignments of the Ministry of Science and Education (No. 0092-2019-0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Fedorova
Rights and permissions
About this article
Cite this article
Agafonov, A.V., Sirotkin, N.A., Titov, V.A. et al. Low-Temperature Underwater Plasma as an Instrument to Manufacture Inorganic Nanomaterials. Russ. J. Inorg. Chem. 67, 253–261 (2022). https://doi.org/10.1134/S0036023622030020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622030020