Abstract
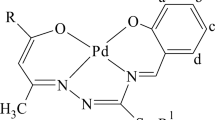
New cationic-anionic palladium(II) complexes have been prepared from adamantyl-substituted imidazolium salts and effect of structure of the adamantyl-substituted salts and synthesis conditions on the structure of these complexes with \({\rm{Pd}}({\rm{DMSO}}){\rm{Hal}}_3^ - \) (1–4), \({\rm{P}}{{\rm{d}}_2}{\rm{Br}}_6^{2 - }\) (5, 6), or \({\rm{PdCl}}_4^{2 - }\) (7) anions has been studied. A number of palladium(II) complexes active against monoamine oxidase B has been prepared, effect of composition and structure on their biological activity have been revealed. The structure of the complexes has been confirmed by X-ray diffraction analysis, a conductometric study of complex 1 has been performed. MAO-inhibiting activity of the obtained complexes has been found to be on the level of reference compounds: 17.6% of residual enzyme activity upon inhibition by complex 3 as compared with 16.9% for reference compound (selegiline). Complexes with bromine ligand show higher activity than those with chlorine ligand. The results of this study can be used in organometallic and bioinorganic chemistry.
Similar content being viewed by others
References
A. Molnar, (Ed.) Palladium-Catalysed Coupling Reactions: Practical Aspects and Future Developments (Wiley-VCH, Weinheim, 2013). doi https://doi.org/10.1002/9783527648283
Li Hongbo, C. C. C. J. Seechurn, and T. J. Colacot, ACS Catal. 2, 1147 (2012). doi https://doi.org/10.1021/cs300082f
S. P. Nolan, N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis (Wiley-VCH, Weinheim, 2014). doi https://doi.org/10.1002/9783527671229
E. S. B. Kantchev, C. J. O’Brien, and M. G. Organ, Angew. Chem., Int. Ed. Engl. 46, 2768 (2007). doi https://doi.org/10.1002/anie.200601663
P. B. Dzhevakov, A. F. Asachenko, M. S. Nechaev, et al., Russ. Chem. Bull. 64, 890 (2014). doi https://doi.org/10.1007/s11172-014-0525-7
W. A. Herrmann, J. Schwarz, and M. G. Gardiner, Organometallics 18, 4082 (1999). doi https://doi.org/10.1021/om990326k
W. A. Herrmann, C. P. Resinger, and M. Spiegler, J. Organomet. Chem. 557, 93 (1998). doi https://doi.org/10.1016/S0022-328X(97)00736-5
C. S. Linningher, E. Herdtweck, S. D. Hoffmann, and W. A. Herrmann, J. Mol. Struct. 890, 192 (2008). doi https://doi.org/10.1016/j.molstruc.2008.05.037
B. Sureshbabu, V. Ramkumar, and S. Sankararaman, Dalton Trans. 43, 10710 (2014). doi https://doi.org/10.1039/C4DT01112K
S. Hohloch, N. Deibel, D. Schweinfurth, et al., Eur. J. Inorg. Chem. 2014, 2131 (2014). doi https://doi.org/10.1002/ejic.201301339
J. C. Bernhammer, N. X. Chong, R. Jothibasu, et al., Organometallics 33, 3607 (2014). doi https://doi.org/10.1021/om500566n
H. V. Huynh and C.-S. Lee, Dalton Trans. 42, 6803 (2013). doi https://doi.org/10.1039/C3DT50237F
M. Heckenroth, E. Kluzer, A. Neels, and M. Albrecht, Angew. Chem., Int. Ed. Engl. 46, 6293 (2007). doi https://doi.org/10.1002/anie.200702199
M. Heckenroth, E. Kluser, A. Neels, and M. Albrecht, Dalton Trans., 6242 (2008). doi https://doi.org/10.1039/B812405A
A. A. Danopoulos, P. Braunstein, N. Stylianides, and M. Wesolek, Organometallics 30, 6514 (2011). doi https://doi.org/10.1021/om200951m
H. Song, N. Yan, Z. Fei, et al., Catalysis Today 183, 172 (2012). doi https://doi.org/10.1016/j.cattod.2011.12.008
Z. Lu and T. J. Williams, ACS Catalysis 6, 6670 (2016). doi https://doi.org/10.1021/acscatal.6b02101
V. Yu. Kukushkin, R. A. Vlasova, and Yu. L. Palzukhina, Zh. Prikl. Khim. 41, 2381 (1968).
A. Hazell, C. J. McKenzie, and L. P. Nielsen, Polyhedron 19, 1333 (2000). doi https://doi.org/10.1016/S0277-5387(00)00409-5
D. Meyer, M. A. Taige, A. Zeller, et al., Organometallics 28, 2142 (2009). doi https://doi.org/10.1021/om8009238
V. V. Sharutin, V. S. Senchurin, and O. K. Sharutina, Russ. J. Inorg. Chem. 58, 543 (2013). doi https://doi.org/10.1134/S0036023613050203
C. Lang, K. Pahnke, C. Kiefer, et al., Polym. Chem. 4, 5466 (2013). doi https://doi.org/10.1039/C3PY90071A
D. Guest, V. H. Menezes Da Silva, A. P. De Lima Batista et al., Organometallics 34, 2463 (2015). doi https://doi.org/10.1021/om5012038
H. V. Huynh, Y. Han, J. H. H. Ho, and G. K. Tan, Organometallics 25, 3267 (2006). doi https://doi.org/10.1021/om060151w
Q.-X. Liu, A.-H. Chen, X.-J. Zhao, et al., CrystEng-Comm 13, 293 (2011). doi https://doi.org/10.1039/C0CE00142B
P. Buchalski, R. Pacholski, K. Chodkiewicz, et al., Dalton Trans. 44, 7169 (2015). doi https://doi.org/10.1039/C4DT03786C
S. K. Yen, L. L. Koh, H. V. Huynh, and T. S. A. Hor, Aust. J. Chem. 62, 1047 (2009). doi https://doi.org/10.1071/CH09196
D. Meyer, M. A. Taige, A. Zeller, et al., Organometallics 28, 2142 (2009). doi https://doi.org/10.1021/om8009238
F. Schroeter, J. Soellner, and T. Strassner, ACS Catal. 7, 3004 (2017). doi https://doi.org/10.1021/acscatal.6b03655
F. Schroeter and T. Strassner, Eur. J. Inorg. Chem. 2017, 4231 (2017). doi https://doi.org/10.1002/ejic.201701000
E. A. Baquero, G. F. Silbestri, P. Gómez-Sal, et al., Organometallics 32, 2814 (2013). doi https://doi.org/10.1021/om400228s
E. A. Baquero, J. C. Flores, J. Perles, et al., Organometallics 33, 5470 (2014). doi https://doi.org/10.1021/om500753v
H. Buhl and C. Ganter, J. Organomet. Chem. 809, 74 (2016). doi https://doi.org/10.1016/j.jorganchem.2016.02.034
E. Borrè, G. Dahm, A. Aliprabdi, et al., Organometallics 33, 4374 (2014). doi https://doi.org/10.1021/om5003446
A. H. Velders, A. Bergamo, E. Alessio, et al., J. Med. Chem. 47, 1110 (2004). doi https://doi.org/10.1021/jm030984d
D. Musumeci, L. Rozza, A. Merlino, et al., Dalton Trans. 44, 13914 (2015). doi https://doi.org/10.1039/C5DT01105A
D. Schleicher, H. Leopold, and T. Strassner, J. Organomet. Chem. 829, 101 (2017). doi https://doi.org/10.1016/j.jorganchem.2016.10.036
A. Gautier and F. Cisnetti, Metallomics 4, 23 (2012). doi https://doi.org/10.1039/c1mt00123j
Ö. Karaca, S. M. Meier-Menches, A. Casini, and F. E. Kühn, Chem. Commun. 53, 8249 (2017). doi https://doi.org/10.1039/C7CC03074F
A. Schmidt, V. Molano, M. Hollering, et al., Chem.-Eur. J. 22, 2253 (2016). doi https://doi.org/10.1002/chem.201504930
A. G. Tikhomirov, N. A. Ivanova, O. S. Erofeeva, et al., Russ. J. Coord. Chem. 29, 489 (2003).
P. B. Gorbacheva, A. G. Tikhomirov, L. Yu. Dederer, et al., Pharm. Chem. J., 42, 53 (2008). doi https://doi.org/10.1007/s11094-008-0058-1
N. A. Kas’yanenko, E. V. Levykina, O. S. Erofeeva, et al., J. Struct. Chem. 50, 996 (2009). doi https://doi.org/10.1007/s10947-009-0148-2
I. A. Efimenko, O. N. Shishilov, N. A. Ivanova, et al., Russ. J. Coord. Chem. 38, 233 (2012). doi https://doi.org/10.1134/S1070328412020029
A. K. Grekhova, L. B. Gorbacheva, N. A. Ivanova, et al., Biochem. Moscow Suppl. Ser. B. 7, 226 (2013). doi https://doi.org/10.1134/S1990750813030050
Y. N. Nosova, I. V. Zenin, V. P. Maximova, et al., Bioinorg. Chem. Appl. 2017, 6 (2017). doi https://doi.org/10.1155/2017/4736321
State Registry of Medicinals (Meditsinskii sovet, Moscow, 2009), Vol. 2, Part 1, p. 568 [in Russian].
M. S. Denisov, Candidate’s Dissertation in Chemistry (Perm, 2015).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009). doi https://doi.org/10.1107/S0021889808042726
G. M. Sheldrick, Acta Crystallogr. A 64, 112 (2008). doi https://doi.org/10.1107/S0108767307043930
G. M. Sheldrick, Acta Crystallogr. C 71, 3 (2015). doi https://doi.org/10.1107/S2053229614024218
Mercury 3.3 (Build RC5), Cambridge Crystallographic Data Centre, 2013. https://doi.org/www.ccdc.cam.ac.uk/mercury/.
U. Thull and B. Testa, Biochem. Pharmacol. 47, 2307 (1994). doi https://doi.org/10.1016/0006-2952(94)90271-2
J. M. Andrade, C. S. Passos, R. R. Dresch, et al., Brazil. Phcog. Mag. 10, s100 (2014). doi https://doi.org/10.4103/09731296.127354
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall, J. Biol. Chem. 193, 265 (1951). https://doi.org/www.jbc.org/content/193/1/265.short.
B. M. F. Gonçalves, J. A. R. Salvador, S. Marín, and M. Cas-cante, Eur. J. Med. Chem. 114, 101 (2016). doi https://doi.org/10.1016/j.ejmech.2016.02.057
E. A. Litvin, G. B. Kolyvanov, and V. P. Zherdev, Farmokinetika i Farmodinamika 1, 18 (2012).
B. Neumüller, S. Chitsaz, and K. Dehnicke, Z. Anorg. Allg. Chem. 628, 523 (2002). doi https://doi.org/10.1002/1521-3749(200203)628:3<523:AID-ZAAC523>3.0.CO;2-C
N. Kuhn, M. Göhner, M. Steimann, and Nachti, Z. Kristallogr. NCS 214, 565 (1999). doi https://doi.org/10.1515/ncrs-1999-0483
X. Wang, Z. Fei, T. J. Geldbach, et al., Organometallics 27, 3971 (2008). doi https://doi.org/10.1021/om800355g
E. Silarska, A. M. Trzeciak, J. Pernak, and A. Skrzypcza, Appl. Catal., A 466, 216 (2013). doi j.apcata.2013.06.046
Z. Huang, F. Li, B. Chen, et al., ChemSusChem 6, 1337 (2013). doi https://doi.org/10.1002/cssc.201300289
C. J. Adams, M. Lusi, E. M. Mutambi, and A. G. Orpen, Chem. Commun. 51, 9632 (2015). doi https://doi.org/10.1039/C5CC02924D
V. V. Sharutin, O. K. Sharutina, V. S. Senchurin, and I. A. Il’chenko, Russ. J. Coord. Chem. 41, 262 (2015). doi https://doi.org/10.1134/S1070328415070088
S. Livingstone, Rhenium, Rhodium, Palladium, Osmium, Iridium, and Platinum (Pergamon, Oxford (UK), 1975), p. 215.
A. K. Lyashchenko, D. V. Loginova, and A. S. Lileev, Russ. J. Coord. Chem. 35, 633 (2009). doi https://doi.org/10.1134/S1070328409090012
H. Huang, N. Humbert, V. Bizet, et al., J. Organomet. Chem. 839, 15 (2017). doi https://doi.org/10.1016/j.jorganchem.2016.12.010
I. A. Efimenko, N. A. Ivanova, O. S. Erofeeva, et al., Russ. J. Coord. Chem. 35, 272 (2009). doi https://doi.org/10.1134/S107032840904007
I. A. Efimenko, A. V. Churakov, N. A. Ivanova, et al., Russ. J. Inorg. Chem. 62, 1469 (2017). doi https://doi.org/10.1134/S0036023617110043
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Denisov, M.S., Dmitriev, M.V., Eroshenko, D.V. et al. Cationic—Anionic Pd(II) Complexes with Adamantylimidazolium Cation: Synthesis, Structural Study, and MAO-Inhibiting Activity. Russ. J. Inorg. Chem. 64, 56–67 (2019). https://doi.org/10.1134/S0036023619010054
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619010054