Abstract
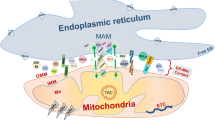
Interactions between the endoplasmic reticulum (ER) and mitochondria have received insufficient attention until recently. However, distorted contacts between the ER and mitochondria were identified as an important factor in the etiopathogenesis of neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. In view of these new data, the mechanisms of ER–mitochondrial interactions are necessary to study in detail in order to develop new diagnostic and therapeutic approaches to neurodegenerative diseases and to extend basic knowledge of the physiology of the eukaryotic cell. The review focuses on the functions of mitochondria-associated ER membranes (MAMs). Structural elements of the MAM system, their contributions to the vital cell functions (calcium and lipid homeostasis, autophagy, fusion and division of mitochondria, and the regulation of their number), and the role of MAM dysfunctions in the pathogenesis of various neurodegenerative diseases are considered.
Similar content being viewed by others
REFERENCES
Skulachev V.P. 2006. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis. 11 (4), 473–485.
Sukhorukov V.S. 2011. Ocherki mitokhondrial’noi patologii (Essays on Mitochondrial Pathology). Moscow. Medpraktika-M.
Wallace D. 2010. Mitochondrial DNA mutations in disease and aging. Environ. Mol. Mutagen. 51 (5), 440–450.
Tsaregorodtsev A.D., Sukhorukov V.S. 2012. Mitochondrial medicine: Problems and tasks. Ross. Vestn. Perinatol. Pediatr. 4 (2), 112–115.
Skulachev M.V., Skulachev V.P. 2014. New data on programmed aging—slow phenoptosis. Biochemistry (Moscow). 70 (10), 977–993.
Hallberg B., Larsson N. 2014. Making proteins in the powerhouse. Cell Metab. 20, 226–240.
Herst P., Rowe M., Carson G., Berridge M.V. 2017. Functional mitochondria in health and disease. Front. Endocrinol. (Lausanne). 8, 296.
Pinton P. 2018. Mitochondria-associated membranes (MAMs) and pathologies. Cell. Death Dis. 9, 413.
Hollien J. 2013. Evolution of the unfolded protein response. Biochim. Biophys. Acta. 1833, 2458–2463.
Bittremieux M., Parys J.B., Pinton P., Bultynck G. 2016. ER functions of oncogenes and tumor suppressors: modulators of intracellular Ca2+ signaling. Biochim. Biophys. Acta. 1863, 1364–1378.
Lai E., Teodoro T., Volchuk A. 2007. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology. 22, 193–201.
Stankov K., Stanimirov B., Mikov M. 2014. Cellular responses to endoplasmic reticulum stress. Biol. Serb. 35, 15–23.
Giorgi C., Missiroli S., Patergnani S., Duszynski J., Wieckowski M.R., Pinton P. 2015. Mitochondria-associated membranes: composition, molecular mechanisms, and physiopathological implications. Antioxid. Redox Signaling. 22, 995–1019.
Bononi A. 2012. Mitochondria-associated membranes (MAMs) as hotspot Ca2+ signaling units. Adv. Exp. Med. Biol. 740, 411–437.
Schreiner B., Ankarcrona M. 2017. Isolation of mitochondria-associated membranes (MAM) from mouse brain tissue. Methods Mol. Biol. 1567, 53–68.
Giacomello M., Pellegrini L. 2016. The coming of age of the mitochondria-ER contact: a matter of thickness. Cell. Death Differ. 23, 1417–1427.
Lahiri S. 2014. A conserved endoplasmic reticulum membrane protein complex (EMC. facilitates phospho-lipid transfer from the ER to mitochondria. PLoS Biol. 12, e1001969.
Kornmann B. 2009. An ER–mitochondria tethering complex revealed by a synthetic biology screen. Science. 325, 477–481.
Kerkhofs M., Bittremieux M., Morciano G. 2018. Emerging molecular mechanisms in chemotherapy: Ca2+ signaling at the mitochondria-associated endoplasmic reticulum membranes. Cell. Death Dis. 9, 334.
ShengnanW., Ming-Hui Z. 2019. Mitochondria-associated endoplasmic reticulum membranes in the heart. Arch. Biochem. Biophys. 662, 201–212.
De Brito O.M., Scorrano L. 2008. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 456, 605–610.
Szabadkai G., Bianchi K. 2006. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 175, 901–911.
Iwasawa R., Mahul-Mellier A.L. 2011. Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J. 30, 556–568.
Stoica R., De Vos K.J., Paillusson S., Mueller S., Sancho R.M., Lau K.F., Vizcay-Barrena G., Lin W.L., Xu Y., Lewis J., Dickson D.W., Petrucelli L., Mitchell J.C., Shaw C.E., Miller C. 2014. ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat. Commun. 5, 3996.
Hirabayashi Y., Kwon S.K., Paek H., Pernice W.M., Paul M.A., Lee J., Efrani P., Raczkowski A., Petrey D.S., Pon L.A., Polleux F. 2017. PZD8 ER-mitochondria tethering by PDZD8 regulates Ca2+ dynamics in mammalian neurons. Science. 358, 623–630.
Wang P.T., Garcin P.O., Wang P.T., Garcin P.O., Fu M., Masoudi M., St-Pierre P., Pante N., Nabi I.R. 2015. Distinct mechanisms controlling rough and smooth endoplasmic reticulum contacts with mitochondria. J. Cell Sci. 128, 2759–2765.
Csordás G., Renken C., Varnai P., Walter L., Weaver D., Buttle K.F., Balla T., Manella C.A., Hajnoczky G. 2006. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 174, 915–921.
Zhang A., Williamson C.D., Wong D.S., Bullough M.D., Brown K.J., Hathout Y., Colberg-Poley A.M. 2011. Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum-mitochondrial contacts at late times of infection. Mol. Cell Proteomics. 10, M111.009936.
Poston C.N., Krishnan S.C., Bazemore-Walker C.R. 2013. In-depth proteomic analysis of mammalian mitochondria-associated membranes (MAM). J. Proteomics. 79, 219–230.
Rizzuto R., Pinton P., Carrington W., Fay F., Fogarty K., Lifshitz L., Tuft R., Pozzan T. 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 280, 1763–1766.
Sood A., Jeyaraju D., Prudent J., Caron A., Lemieux P., McBride H., Laplante M., Toth K., Pellegrini L. 2014. A mitofusin-2-dependent inactivating cleavage of Opa1 links changes in mitochondria cristae and ER contacts in the postprandial liver. Proc. Natl. Acad. Sci. U. S. A. 111, 16017–16022.
Bootman M.D. 2012. Calcium signaling. Cold Spring Harb. Perspect. Biol. 4, a011171.
Clapham D.E. 2007. Calcium signaling. Cell. 131, 1047–1058.
Tadini-Buoninsegni F., Smeazzetto S. 2018. Drug interactions with the Ca2+-ATPase from sarco(endo)plasmic reticulum (SERCA). Front. Mol. Biosci. 20, 123–136.
Chemaly E.R., Troncone L., Lebeche D., Smeazzetto S., Gualdani R., Moncelli M. 2018. SERCA control of cell death and survival. Cell Calcium. 69, 46–61.
Stefani D., Rizzuto R., Pozzan T. 2016. Enjoy the trip: Calcium in mitochondria back and forth. Annu. Rev. Biochem. 85, 161–192.
Bononi A., Missiroli S., Poletti F., Suski J., Agnoletto C., Bonora M., Marchi E., Giorgi C., Marchi S., Patergnani S., Wieckowski M., Pinton P. 2012. Mitochondria-associated membranes (MAMs) as hotspot Ca2+ signaling units. Adv. Exp. Med. Biol. 740, 411–437.
Veeresh P., Kaur H., Sarmah D., Mounica L., Verma G., Kotian V., Kesharwani R., Kalia K., Borah A., Wang X., Dave K., Rodriguez AM, Yagaval D., Bhattacharya P. 2019. Endoplasmic reticulum–mitochondria crosstalk: From junction to function across neurological disorders. Ann. N. Y. Acad. Sci. 1457, 41–60.
Parys J.B., De Smedt H. 2012. Inositol 1,4,5-trisphosphate and its receptors. Adv. Exp. Med. Biol. 740, 255–279.
Fekete A., Nakamura Y., Yang Y., Herlitze S., Mark M., DiGregorio D., Wang L. 2019. Underpinning heterogeneity in synaptic transmission by presynaptic ensembles of distinct morphological modules. Nat. Commun. 10, 826.
Mishra P., Chan D.C. 2016. Metabolic regulation of mitochondrial dynamics. Cell Biol. 212, 379–387.
Tagaya M., Arasaki K. 2017. Regulation of mitochondrial dynamics and autophagy by the mitochondria-associated membrane. Adv. Exp. Med. Biol. 997, 33–47.
Schrepfer E., Scorrano L. 2016. Mitofusins, from mitochondria to metabolism. Mol. Cell. 61, 683–694.
Zorzano A., Hernández-Alvarez M.I., Sebastian D., Munoz J.P. 2015. Mitofusin 2 as a driver that controls energy metabolism and insulin signaling. Antioxid. Redox Signal. 22, 1020–1031.
Dorn G.W., Song M., Walsh K. 2015. Functional implications of mitofusin 2-mediated mitochondrial–SR tethering. J. Mol. Cell Cardiol. 78, 123–128.
Anand R., Wai T., Baker M., Kladt N., Schauss A., Rugarli E., Langer T. 2014. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 204, 919–929.
Richter V., Palmer C.S., Osellame L., Singh A., Elgass K., Stroud D., Sesaki H., Kvansakul M., Ryan M. 2014. Structural and functional analysis of MiD51, a dynamin receptor required for mitochondrial fission. J. Cell Biol. 204, 477–486.
Jin X., Wang J. 2017. Dysregulation of INF2-mediated mitochondrial fission in SPOP-mutated prostate cancer. PLoS Genet. 13, e1006748.
Wikstrom J.D., Mahdaviani K., Liesa M., Sereda S.B., Si Y., Las G., Twig G., Petrovic N., Zingaretti C., Graham A., Cinti S., Corkey B., Cannon B., Nedergaard J., Shirihai O. 2014. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J. 33, 418–436.
Sheng Z.H. 2017. The interplay of axonal energy homeostasis and mitochondrial trafficking and anchoring. Trends Cell Biol. 27, 403–416.
Hailey D.W., Rambold A.S. 2010. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 141, 656–667.
Hamasaki M., Furuta N., Matsuda A., Nezu A., Yamamoto A., Fujita N., Hiroko O., Noda T., Haraguchi T., Hiraoka Y., Amano A., Yoshimori T. 2013. Autophagosomes form at ER-mitochondria contact sites. Nature. 495, 389–393.
Arasaki K., Shimizu H., Mogari H., Nishida N., Hirota N., Furuno A., Kudo Y., Baba M., Baba N., Cheng J., Furuta N., Matsuda A., Nezu A., Yamamoto A., Fujita N., et al. 2013. A role for the ancient SNARE syntaxin 17 in regulating mitochondrial division. Dev. Cell. 32, 304–317.
Walter P., Ron D. 2011. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 334, 1081–1086.
Lan B., He Y., Sun H., Zheng X., Gao Y., Li N. 2019. The roles of mitochondria-associated membranes in mitochondrial quality control under endoplasmic reticulum stress. Life Sci. 231, 116587.
Glancy B., Balaban R.S. 2012. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 51 (14), 2959–2973. doi https://doi.org/10.1021/bi2018909
Son S.M., Byun J., Roh S.E., Kim S.J., Mook-Jung I. 2014. Reduced IRE1α mediates apoptotic cell death by disrupting calcium homeostasis via the InsP3 receptor. Cell. Death Dis. 5 (4), 1188. doi https://doi.org/10.1038/cddis.2014.129
Rambold A.S., Kostelecky B., Elia N., Lippincott-Schwartz J. 2011. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl. Acad. Sci. U. S. A. 108, 10190–10195.
Zhang Y., Ren S., Liu Y., Gao K., Liu Z., Zhang Z. 2017. Inhibition of starvation-triggered endoplasmic reticulum stress, autophagy, and apoptosis in ARPE-19 cells by taurine through modulating the expression of calpain-1 and calpain-2. Int. J. Mol. Sci. 18, 23–24.
Cui J., Li Z., Zhuang S., Qi S., Li L., Zhou J., Zhang W., Zhao Y. 2018. Melatonin alleviates inflammation-induced apoptosis in human umbilical vein endothelial cells via suppression of Ca2+-XO-ROS-Drp1-mitochondrial fission axis by activation of AMPK/SERCA2a pathway. Cell Stress Chaperones. 23, 281–293.
Gelmetti V., De Rosa P., Torosantucci L., Marini E.S., Romagnoli A., Di Rienzo M., Arena G., Vignone D., Fimia G.M., Valente E.M. 2017. PINK1 and BECN1 relocalize at mitochondria-associated membranes during mitophagy and promote ER-mitochondria tethering and autophagosome formation. Autophagy. 13 (4), 654–669.
Deniaud A., Sharaf el dein O., Maillier E., Poncet D., Kroemer G., Lemaire C., Brenner C. 2008. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 27, 285–299.
Vervliet T., Clerix E., Seitaj B., Ivanova H., Monaco G., Bultynck G. 2017. Modulation of Ca2+ signaling by anti-apoptotic B-cell lymphoma 2 proteins at the endoplasmic reticulum-mitochondrial interface. Front. Oncol. 7, 75–76.
Oakes S.A., Scorrano L., Opferman J.T., Bassik M.C., Nishino M., Pozzan T., Korsmeyer S.J. 2005. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 102, 105–110.
Monaco G., Decrock E., Arbel N., van Vliet A.R., La Rovere R.M., De Smedt H., Parys J.B., Agostinis P., Leybaert L., Shoshan-Barmatz V., Bultynck G. 2015. The BH4 domain of anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the voltage-dependent anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria. J. Biol. Chem. 290, 9150–9161.
Banerjee J., Ghosh S. 2004. Bax increases the pore size of rat brain mitochondrial voltage-dependent anion channel in the presence of tBid. Biochem. Biophys. Res. Commun. 323, 310–314.
Sassano M.L., van Vliet A.R., Agostinis P. 2017. Mitochondria-associated membranes as networking platforms and regulators of cancer cell fate. Front. Oncol. 7, 174.
Dietel E., Brobeil A., Delventhal L., Tag C., Gattenlohner S., Wimmer M. 2019. Crosstalks of the PTPIP51 interactome revealed in Her2 amplified breast cancer cells by the novel small molecule LDC3/Dynarrestin. PLoS One. 14 (5), e0216642.
Herrera-Cruz M.S., Simmen T. 2017. Cancer: untethering mitochondria from the endoplasmic reticulum? Front. Oncol. 7, 105.
Janikiewicz J., Hanzelka K., Kozinski K., Kolczynska K., Dobrzyn A. 2015. Islet beta-cell failure in type 2 diabetes – within the network of toxic lipids. Biochem. Biophys. Res. Commun. 460, 491–496.
Szymański J., Janikiewicz J., Michalska B., Patalas-Krawczyk P., Perrone M., Ziółkowski W., Duszyński J., Pinton P., Dobrzyń A., Więckowski M. R. 2017. Interaction of mitochondria with the endoplasmic reticulum and plasma membrane in calcium homeostasis, lipid trafficking and mitochondrial structure. Int. J. Mol. Sci. 18, 1576.
Tubbs E., Rieusset J. 2016. Metabolic signaling functions of ER-mitochondria contact sites: Role in metabolic diseases. Soc. Endocrinol. 58, 87–R106.
Thivolet C., Vial G., Cassel R., Rieusset J., Madec A.M. 2017. Reduction of endoplasmic reticulum–mitochondria interactions in beta cells from patients with type 2 diabetes. PLoS One. 12, e0182027.
Tubbs E., Chanon S., Robert M., Benridi N., Bidaux G., Chauvin M.A., Ji-Cao J., Durand C., Gayrit-Ramette D., Vidal H., Lefai E., Rieusset J. 2018. Disruption of mitochondria-associated endoplasmic reticulum membrane (MAM) integrity contributes to muscle insulin resistance in mice and humans. Diabetes. 67, 636–650.
Tubbs E., Theurey P., Vial G., Bendridi N., Bravard A., Chauvin M.A., Ji-Cao J., Zoulim F., Bartosch B., Ovize M., Vidal H., Rieusset J. 2014. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes. 63, 3279–3294.
Sasi U.S.S., Ganapathy S., Palayyan S.R., Gopal R.K. 2020. Mitochondria associated membranes (MAMs): Emerging drug targets for diabetes. Curr. Med Chem. 27, 3362–3385.
Shinjo S., Jiang S., Nameta M., Suzuki T., Kanai M., Nomura Y., Goda N. 2017. Disruption of the mitochondria-associated ER membrane (MAM) plays a central role in palmitic acid-induced insulin resistance. Exp. Cell Res. 359, 86–93.
Burgos-Moron E., Abad-Jimenez Z., Maranon A.M., Iannantuoni F., Escribano-Lopez I., Lopez-Domenech S., Salom C., Jover A., Mora V., Roldan I. 2019. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 8, 1385.
Rodríguez-Arribas M., Yakhine-Diop S.M.S., Pedro J.M.B., Gomez-Suaga P., Gomez-Sanchez R., Martinez-Chacon G., Fuentes J.M., Gonzalez-Polo R.A., Niso-Santano M. 2017. Mitochondria-associated membranes (MAMs): Overview and its role in Parkinson’s disease. Mol. Neurobiol. 54, 6287–6303.
Haile Y., Deng X., Ortiz-Sandova C., Tahbaz N., Janowicz A., Lu J.-Q., Kerr B.J., Gutowski N.J., Holley J.E., Eggleton P., Giuliani F., Simmen T. 2017. Rab32 connects ER stress to mitochondrial defects in multiple sclerosis. J. Neuroinflammation. 14, 19.
Delfina L., Pera M., Gonnelli, Quintana-Cabrera R., Akman H.O, Guardia-Laquarta C., Velasco K.R., Area-Gomez E., Dal Bello F., Stefani D., Horvath R., Shy M., Schon M., Giacomello M. 2019. MFN2 mutations in Charcot–Marie–Tooth disease alter mitochondria-associated ER membrane function but do not impair bioenergetics. Hum. Mol. Genetics. 28, 1782–1800.
Paillusson S., Stoica R., Gomez-Suaga P., Lau D.H.W., Mueller S., Miller T., Miller C.C.J. 2016. There’s something wrong with my MAM; the ER-mitochondria axis and neurodegenerative diseases. Trends Neurosci. 39, 146–157.
Manfredi G., Kawamata H. 2016. Mitochondria and endoplasmic reticulum crosstalk in amyotrophic lateral sclerosis. Neurobiol. Dis. 90, 35–42.
Reijonen S., Putkonen N., Norremolle A., Lindholm D., Korhonen L. 2008. Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant Huntingtin proteins. Exp. Cell Res. 314, 950–960.
Eysert F., Kinoshita P.F., Mary A., Vaillant-Beuchot L., Checler F., Chami M. 2020. Molecular dysfynctions of mitochondria-associated membranes (MAMs) in Alzheimer’s disease. Int. J. Mol. Sci. 21(24), 9521.
Hyrskyluoto A., Pulli I., Tornqvist K., Ho TH., Korhonen L., Lindholm D. 2013. Sigma-1 receptor agonist PRE084 is protective against mutant Huntingtin-induced cell degeneration: Involvement of calpastatin and the NF-kappaB pathway. Cell Death Dis. 4, e646.
Penke B., Fulop L., Szucs M., Frecska E. 2018. The role of sigma-1 receptor, an intracellular chaperone in neurodegenerative diseases. Curr. Neuropharmacol. 16, 97.
Ryskamp DA., Korban S., Zhemkov V., Kraskovskaya N., Bezprozvanny I. 2019. Neuronal sigma-1 receptors: signaling functions and protective roles in neurodegenerative diseases. Front. Neurosci. 13, 862.
Hayashi T., Su T.P. 2007. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 131, 596–610.
Zarei S., Carr K., Reiley L., Diaz K., Guerra O., Altamirano P. F., Pagani W., Lodin D., Orozco G., Chinea A. 2015. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 6, 171.
Ryan B.J., Hoek S., Fon EA., Wade-Martins R. 2015. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem. Sci. 40, 200–210.
Apicco D.J., Shlevkov E., Nezich C.L., Tran D.T., Guilmette E., Nicholatos J.W., Bantle C.M., Chen Y., Glajch K.E., Abraham N.A., Dang L.T., Kaynor G.C., Tsai E.A., Nguyen K.H., Groot J., et al. 2021. The Parkinson’s disease-associated gene ITPKB protects against α-synuclein aggregation by regulating ER-to-mitochondria calcium release. Proc. Natl. Acad. Sci. U. S. A. 118 (1), e2006476118.
Sukhorukov V.S., Voronkova A.S., Litvinova N.A., Baranich T.I., Illarioshkin S.N. 2020. The role of mitochondrial DNA individuality in the pathogenesis of Parkinson’s disease. Russ. J. Genet. 56 (4), 402–409.
Ozcan L., Tabas I. 2012. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev. Med. 63, 317–328.
Gómez-Suaga P., Pedro J.M., González-Polo R.A., Fuentes J., Niso-Santano M. 2018. ER–mitochondria signaling in Parkinson’s disease. Cell Death Dis. 9, 337.
Guardia-Laguarta C., Area-Gomez E., Rub C., Liu Y., Magrane J., Becker D., Voos W., Schon E.A., Przedborski S. 2014. Alpha-synuclein is localized to mitochondria-associated ER membranes. J. Neuroscience. 34, 249–259.
Cali T., Ottolini D., Negro A., Brini M. 2012. Alpha-synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum–mitochondria interactions. J. Biol. Chem. 287, 17914–17929.
Sun X., Liu J., Crary J.F., Malagelada C., Sulzer D., Greene L.A., Levy O.A. 2013. ATF4 protects against neuronal death in cellular Parkinson’s disease models by maintaining levels of parkin. J. Neurosci. 33, 2398–2407.
Bouman L., Schlierf A., Lutz A.K., Shan J., Deinlein A., Kast J., Galehdar Z., Palmisano V., Patenge N., Berg D., Gasser T., Augustin R., Trumbach D., Irrcher I., Park D.S., et al. 2011. Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ. 18, 769–782.
Cali T., Ottolini D., Negro A., Brini M. 2013. Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca2+ transfer to sustain cell bioenergetics. Biochim. Biophys. Acta. 4, 495–508.
Wu S., Lei L., Song Y., Liu M., Lu S., Lou S., Shi Y., Wang Z., He D. 2018. Mutation of hop-1 and pink-1 attenuates vulnerability of neurotoxicity in C. elegans: the role of mitochondria-associated membrane proteins in Parkinsonism. Exp. Neurology. 309, 67–78.
Ottolini D., Cali T., Negro A., Brini M. 2013. The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering. Hum. Mol. Genet. 11, 2152–2168.
Gómez-Suaga P., Bravo-San Pedro J.M., González-Polo R.A., Fuentes JM., Nino-Santano M. 2018. ER–mitochondria signaling in Parkinson’s disease. Cell Death Dis. 9, 337.
Sun D., Chen X., Gu G., Wang J., Zhang J. 2017. Potential roles of mitochondria-associated ER membranes (MAMs) in traumatic brain injury. Cell. Mol. Neurobiol. 37(8), 1349–1357.
Marchi S., Bittremieux M., Missiroli S., Morganti C., Patergnani S., Sbano L., Rimessi A., Kerkhofs M., Parys J.B., Bultynck G., Giorgi C., Pinton P. 2017. Endoplasmic reticulum-mitochondria communication through Ca2+ signaling: the importance of mitochondria-associated membranes (MAMs). Adv. Exp. Med. Biol. 997, 49–67.
Watanabe S., Ilieva H., Tamada H., Nomura H., Komine O., Endo F., Jin S., Mancias P., Kiyama H., Yamanaka K. 2016. Mitochondria-associated membrane collapse is a common pathomechanism in SIGMAR1-and SOD1- linked ALS. EMBO Mol. Med. 8, 1421–1437.
Yonashiro R., Sugiura A., Miyachi M., Fukuda T., Matsushita N., Inatome R., Ogata Y., Suzuki T., Dohmae N., Yanagi S. 2009. Mitochondrial ubiquitin ligase MITOL ubiquitinates mutant SOD1 and attenuates mutant SOD1-induced reactive oxygen species generation. Mol. Biol. Cell. 20, 4524–4530.
Nishimura A.L., Mitne-Neto M., Silva H.C.A., Richieri-Costa A., Middleton S., Cascio D., Kok F., Oliveira J.R.M., Gillingwater T., Webb J., Skehel P., Zatz M. 2004. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am. J. Hum. Genet. 75, 822–831.
Anagnostou G., Akbar M.T., Paul P., Angelinetta C., Steiner T.J., de Belleroche J. 2014. Vesicle associated membrane protein B (VAPB) is decreased in ALS spinal cord. Neurobiol. Aging. 31, 969–985.
Kim J.Y., Jang A., Reddy R., Yoon W.H., Jankowsky J.L. 2016. Neuronal overexpression of human VAPB slows motor impairment and neuromuscular denervation in a mouse model of ALS. Hum. Mol. Genet. 25, 4661–4673.
Haile Y., Deng X., Ortiz-Sandova C., Tahbaz N., Janowicz A., Lu J-Q., Kerr B.J., Gutowski N.J., Holley J.E., Eggleton P., Giuliani F., Simmen T. 2017. Rab32 connects ER stress to mitochondrial defects in multiple sclerosis. J. Neuroinflam. 14, 19.
Völgyi K., Badics K., Sialana F.J., Gulyassy P., Udvari E.B., Kis V., Drahos L., Lubec G., Kekesi K.A., Juhasz G. 2018. Early presymptomatic changes in the proteome of mitochondria-associated membrane in the APP/PS1 mouse model of Alzheimer’s disease. Mol. Neurobiol. 55, 7839–7857.
Contino S., Porporato P.E., Bird M., Marinangeli C., Opsomer R., Sonveaux P., Bontemps F., Dewachter I., Octave J.-N., Bertrand L., Stanga S., Kienlen-Campard P. 2017. Presenilin 2-dependent maintenance of mitochondrial oxidative capacity and morphology. Front. Physiol. 8, 796.
Zampese E., Fasolato C., Kipanyula M.J., Bortolozzi M., Pozzan T., Pizzo P. 2011. Presenilin 2 modulates endoplasmic reticulum (ER)–mitochondria interactions and Ca2+ cross-talk. Proc. Natl. Acad. Sci. U. S. A. 108, 2777–2782.
Erpapazoglou Z., Mouton-Liger F., Corti O. 2017. From dysfunctional endoplasmic reticulum–mitochondria coupling to neurodegeneration. Neurochem. Int. 109, 171–183.
De Brito O.M., Scorrano L. 2008. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 456, 605–610.
Area-Gomez E., Del Carmen Lara Castillo M., Tambini M.D., Guardia-Laguarta C., de Groof A.J., Madra M., Ikenouchi J., Umeda M., Bird T.D., Sturley S.L., Schon E.A. 2012. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 31, 4106–4123.
Voelker D.R. 2005. Bridging gaps in phospholipid transport. Trends Biochem. Sci. 30, 396–404.
Area-Gomez E., Schon E.A. 2016. Mitochondria-associated ER membranes and Alzheimer disease. Curr. Opin. Genet. Dev. 38, 90–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving humans or animals performed by any of the authors.
Additional information
Translated by T. Tkacheva
Rights and permissions
About this article
Cite this article
Sukhorukov, V.S., Voronkova, A.S., Baranich, T.I. et al. Molecular Mechanisms of Interactions between Mitochondria and the Endoplasmic Reticulum: A New Look at How Important Cell Functions are Supported. Mol Biol 56, 59–71 (2022). https://doi.org/10.1134/S0026893322010071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893322010071