Abstract
Here, we discuss pathophysiological approaches to the defining of endothelial dysfunction criteria (i.e., endothelial activation, impaired endothelial mechanotransduction, endothelial-to-mesenchymal transition, reduced nitric oxide release, compromised endothelial integrity, and loss of anti-thrombogenic properties) in different in vitro and in vivo models. The canonical definition of endothelial dysfunction includes insufficient production of vasodilators, pro-thrombotic and pro-inflammatory activation of endothelial cells, and pathologically increased endothelial permeability. Among the clinical consequences of endothelial dysfunction are arterial hypertension, macro- and microangiopathy, and microalbuminuria. We propose to extend the definition of endothelial dysfunction by adding altered endothelial mechanotransduction and endothelial-to-mesenchymal transition to its criteria. Albeit interleukin-6, interleukin-8, and MCP-1/CCL2 dictate the pathogenic paracrine effects of dysfunctional endothelial cells and are therefore reliable endothelial dysfunction biomarkers in vitro, they are non-specific for endothelial cells and cannot be used for the diagnostics of endothelial dysfunction in vivo. Conceptual improvements in the existing methods to model endothelial dysfunction, specifically, in relation to the blood–brain barrier, include endothelial cell culturing under pulsatile flow, collagen IV coating of flow chambers, and endothelial lysate collection from the blood vessels of laboratory animals in situ for the subsequent gene and protein expression profiling. Combined with the simulation of paracrine effects by using conditioned medium from dysfunctional endothelial cells, these flow-sensitive models have a high physiological relevance, bringing the experimental conditions to the physiological scenario.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The study of endothelial physiology continues to be among the most relevant topics in cardiovascular biology, as evidenced by the steady increase in relevant publications over the past 20 years (from 9,512 in 2001 to 21,748 in 2021). Of particular relevance is the study of endothelial dysfunction, as the proportion of relevant publications across all publications on endothelial physiology is growing every year (from 5.99% in 2001 to 10.83% in 2021). Interest in endothelial dysfunction in the last two years (Fig. 1) can be explained by the COVID-19 pandemic, as this disease is accompanied by endothelial dysfunction in a significant proportion of patients.
Despite a significant number of publications on endothelial dysfunction in the leading cardiovascular journals (Circulation Research, Cardiovascular Research, Arteriosclerosis, Thrombosis, and Vascular Biology) over the past 20 years (671), which deciphered a number of mechanisms either ensuring normal physiology of the endothelium (maintaining the endothelial phenotype, as well as the arterial, venous or lymphatic specification of the endothelium, the protective effect of laminar flow with high shear stress) or contributing to its pathological activation (atherogenic effects of turbulent flow, endothelial-to-mesenchymal transition, impaired endothelial mechanotransduction), the definition of endothelial dysfunction has remained static since the 1990s. In both descriptive and critical reviews, endothelial dysfunction is defined as “reduced synthesis, release or bioavailability of nitric oxide (NO), which can lead to an imbalance between vasoconstriction and vasodilation and is accompanied by pro-inflammatory and prothrombotic activation of the endothelium” [1–6]. More concise definitions of endothelial dysfunction sound like “impairment of endothelium-dependent vasodilation in response to physiological stimuli” [7, 8] or “loss of anti-inflammatory, antithrombotic and vasodilating capacity of the endothelium” [9]. A clinically relevant definition of endothelial dysfunction is “reduced vasodilatory response to an endothelium-dependent vasodilator (e.g., acetylcholine or bradykinin) or impaired flow-mediated vasodilation” [10]. Perhaps the most complete definition of endothelial dysfunction today belongs to M.S. Goligorsky, who proposed to include impaired barrier function, compromised proliferation and migration, and decreased angiogenic potential of dysfunctional endothelial cells into the endothelial dysfunction definition [11, 12]. Moreover, he denoted the clinical consequences of endothelial dysfunction: arterial hypertension, macro- and microangiopathy, which both develop as a result of atherogenesis and inflammatory infiltration, and microalbuminuria, which occurs due to increased vascular permeability [11, 12]. He postulated the following signatures of dysfunctional endothelial cells: reduced release of bioavailable NO, increased ability to leukocyte adhesion, accumulation of cholesterol and oxidized low-density lipoproteins, impaired transmission of vasodilating molecules to vascular smooth muscle cells, increased synthesis of extracellular matrix components (acquisition of a profibrotic phenotype) and a tendency to premature aging and apoptosis [11, 12].
At the same time, the work of joint research group to determine the clinical and laboratory criteria of endothelial dysfunction did not lead to a satisfactory result according to its own conclusion [13]. Almost all biomarkers from 62 studies assessed were characterized by a lack of specificity for endothelial cells [13]. The authors of a large-scale proteomic study to identify markers associated with endothelial dysfunction in patients with diabetes mellitus came to similar results [14]. Thus, both pathophysiological and clinical definitions of endothelial dysfunction are unclear, and the classical definition of this concept is limited to the impairment of endothelium-dependent vasorelaxation. Some authors expand one of the endothelial dysfunction components to “dysfunctional release of messenger molecules” without being restricted to NO [15]. A significant amount of new data on endothelial physiology of the endothelium in the last 15 years requires introducing novel criteria for endothelial dysfunction. This article is devoted to a critical discussion of various aspects of endothelial physiology, including those important for the proper modeling of the blood–brain and blood-air barriers, in a methodological context.
METHODOLOGICAL ASPECTS OF MODELING ENDOTHELIAL DYSFUNCTION
Endothelial cell culture under flow
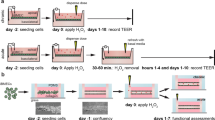
Unlike most cell lines, three-dimensional culturing of which under static conditions relatively corresponds to the biomechanical effects they experience in the body, endothelial cells require the creation of a unidirectional pulsating flow in order to adequately model their physiology and particularly gene and protein expression profiles. A suitable solution for culturing endothelial cells under flow conditions is a system that combines a flow pump, tubing connectors, cell culture chambers, and software to control flow settings (including shear stress). The advantages of using such a system are the variability of available connectors and culture flow chambers, which can be coated with type IV collagen for imitation of the basement membrane. The transparency of the flow chambers allows visualization of viable or fixed endothelial cell cultures at any time using phase-contrast, epifluorescence or confocal microscopy after labeling with fluorescent probes (for the viable cultures) or fluorophore-conjugated antibodies (for the fixed cultures). The possibility of fluorescence visualization of viable cell cultures makes it possible to study lysosome functioning (as lysosomal probes are pH sensors, the pH gradient and lysosome staining are often lost during fixation) and mitochondria without the need to stain these organelles with specific antibodies. The presence of four separate modules connected to one pump and controlled by specialized software via a laptop, allows simultaneous work with four experimental groups and permits maneuvering when planning the design of the experiment. The analysis is usually performed after a temporary shutdown of the flow system and removal of the flow chambers from it; however, the length of the connectors allows cell visualization under flow if necessary.
An important technical nuance of working with a flow system is the endothelial cell confluence in flow chambers to ensure the endothelial integrity during the experiment. In order to provide a reliable adhesion to the culture plastic (or type IV collagen if needed), after the passaging into flow chambers cells should be left for 12–16 h under static conditions. In order to adapt endothelial cells to the flow conditions, it is recommended to precondition the adhered endothelial cells by placing the flow chamber into a pulsating flow system for 24–48 h without any additional stimuli. The criterion for cell adaptation to the flow, which indirectly reflects the corresponding changes in the initial gene and protein expression, is the elongated shape of endothelial cells.
It should be noted that the full confluence of the endothelial monolayer prevents the proper elongation of endothelial cells, which can reduce the physiology of modeling mechanotransduction (transformation of mechanical cues such as flow into biochemical signals), but at the same time ensures endothelial integrity which is no less important for an adequate assessment of its physiology. On the contrary, incomplete confluence of the endothelial monolayer allows endothelial cells to sufficiently change the geometry in accordance with the direction of flow; however, it also leaves the possibility of impaired endothelial integrity, which can lead to the detachment of endothelial cells from the chamber. As a rule of thumb, a 75–80% confluence is optimal for the physiological modeling of endothelial mechanotransduction and, consequently, for the assessment of gene and protein expression profile.
The amount of endothelial cells within one flow culture chamber is sufficient for the gene expression analysis by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) upon the RNA extraction. If whole transcriptome sequencing (RNA-seq), liquid chromatography with tandem mass spectrometry (LC-MS) or Western blotting is required, it is recommended to pool cell lysate from three flow chambers within one experimental group into one sample to obtain a sufficient material for analysis.
When cells inside the flow chamber are lysed with Trizol (for RNA isolation) or RIPA buffer (for protein isolation), it is important to withdraw the maximum amount of phosphate-buffered saline (used for cell washing) from the chamber (leaving it only inside the chamber channel itself). Due to the different density of Trizol, RIPA buffer and phosphate-buffered saline, trizol and RIPA-buffer practically do not mix with phosphate-buffered saline and displace it from the channel to the outside of the chamber within a few seconds (while pipetting is in progress), which makes it possible to remain the residual phosphate-buffered saline in the chamber in order to avoid air embolism of the channel. After a 5-min cell lysis in Trizol or RIPA buffer, it is recommended to pass the Trizol or RIPA buffer collected from the chamber through the chamber twice again to collect the remaining cell debris.
For culturing endothelial cells under flow conditions, it is advisable to use a complete (serum-containing) culture medium. A complete medium is most suitable for the settlement of endothelial cells in flow culture chambers and for their preconditioning under flow. Conceivably, such medium can be used during the flow experiments; however, it can also be replaced with a serum-free culture medium (as serum is a stochastic combination of heterogeneous molecules that might impact the experiments). From this point of view, serum-free media with a predetermined concentration of growth factors (defined medium) are of significant interest. Defined medium makes it possible both to increase the viability of endothelial cells during the experiment and to minimize the stochastic effect of heterogeneous serum on the results. A wide range of media for culturing primary human endothelial cells is currently available on the market, including serum-containing, serum-free, and defined medium. Importantly, media from different manufacturers and the corresponding primary endothelial cultures are interchangeable.
Further, the importance of using primary rather than immortalized cultures should be emphasized. It is expedient to purchase commercial cultures of endothelial cells which are capable of rapid proliferation (the rate depends on the donor and on the cell line, but in some cases is not inferior to immortalized endothelial cells), are easy for subculturing and withstand multiple passages (thawed at the 2nd passage, in our hands it was possible to subculture them up to the 12th passage without a significant slowdown in proliferation). Such an approach to endothelial cell sourcing makes it possible to select the cell line of virtually every blood vessel. To model endothelial dysfunction in atherosclerosis, we recommend performing simultaneous experimentation on endothelial cells from atherosensitive arteries (e.g., the coronary artery, carotid artery, or aorta) and atheroresistant arteries (such as internal thoracic artery). The use of arterial endothelial cells is mandatory to study arterial diseases such as atherosclerosis, restenosis or aneurysms, while venous endothelial cells are required to study venous pathologies such as venous thromboembolism. It is worth focusing to study the physiology of endothelial cells that populate novel biomaterials for the production of tissue-engineered medical devices (e.g., small-diameter vascular grafts or bioprosthetic heart valves). To model the (patho)physiology of the endothelium in such conditions, one should also use endothelial cells lines that correspond to the anatomical localization of the medical device (e.g., if small-diameter vascular prostheses are planned to be used as conduits for coronary bypass grafting, experiments to study endothelial physiology on such conduits or biomaterials for them should be carried out on the primary coronary or internal thoracic artery endothelial cells).
Endothelial cell culture under static conditions
Despite the attractiveness of culturing endothelial cells under pulsating flow conditions, a number of experimental applications require a significant amount of cells, which is impossible to be obtained in a small culture area of flow chambers. For example, the analysis of paracrine effects of endothelial cells necessarily includes the measurement of pro-inflammatory cytokines, which concentration must be sufficient for their detection. The volume of the culture medium loaded into one pulsed flow module (utilizing the system used by the authors as an example) is 15 mL, and the area of one culture flow chamber is » 2.5–3.0 cm2, which is equivalent to the area of 8–9 wells of a 96-well culture plate or 0.25–0.30 ´ 106 (250,000–300,000) cells. Accordingly, the number of cells cultured in the flow system does not correspond to the volume of the medium in terms of even high-sensitive enzyme-linked immunosorbent assay. Evaporation of the culture medium to concentrate the molecules is associated with a high risk of contamination, which makes impossible any further analysis. In addition, modeling the paracrine effects of endothelial cells requires the addition of conditioned (i.e., incubated with dysfunctional endothelial cells) medium to intact cultures, which again seems unrealistic under flow conditions due to the discrepancy between the number of cells and the volume of the medium.
Therefore, experiments on endothelial cell secretome (cytokines, pro- and anti-angiogenic molecules) are advisable to be carried out under static conditions. When modeling the paracrine effects of endothelial cell cultures, one should remember the importance of using a serum-free or defined culture medium as the serum contains xenogeneic (heterologous) extracellular vesicles as well as cytokines and pro-/anti-angiogenic molecules which may interact with polyclonal antibodies for enzyme-linked immunosorbent assay or dot blotting. The use of a defined medium increases the viability of cells exposed to an endothelial dysfunction trigger yet making it difficult to objectively assess changes in pro- and anti-angiogenic molecules. As a rule, the conditioned medium is transferred to intact endothelial cell cultures after no more than 24 h of cultivation to prevent further changes in endothelial physiology associated with serum deprivation. In addition, to objectively assess the response of intact endothelial cell cultures to conditioned medium (including changes in their secretory profile), their 24-h incubation with a conditioned medium is required. In such experimental setting, culture medium added to an endothelial cell monolayer becomes depleted just after 48 hours (24 h of incubation with dysfunctional cells and 24 h of incubation with intact cell cultures).
Also, endothelial cell culture under static conditions is applicable when the experiment involves their fractionation into organelles in order to separate proteins of different localization (e.g., nuclear, cytosolic and mitochondrial or lysosomal proteins). Further applications in this design may include liquid chromatography with tandem mass spectrometry, which requires pre-enrichment of the analyzed samples to maximize the total number of identifiable cellular proteins. This requires a significant number of endothelial cells to collect sufficient protein amounts that is impossible to be grown at flow culture chambers.
Study of endothelial biology in vivo
In addition to in vitro experiments on endothelial cell cultures under unidirectional flow conditions, studies of pathological effects and their mechanisms also involve in vivo studies on laboratory animals. Small laboratory animals (rats and mice) can be considered the most suitable models for this due to a relative simplicity of their housing and reproduction, which makes possible to conduct large-scale experiments with a sufficient number of biological replicates.
Considering possible animal models for studying endothelial dysfunction, it should be emphasized that each experiment may require individualization of the experimental model (e.g., certain strains of transgenic mice or rats). This postulate is especially applicable to the analysis of the impact of various comorbid conditions, e.g., diabetes mellitus, chronic kidney disease, and chronic obstructive pulmonary disease. Each of these diseases has its specific, both conventional and novel animal models. If considering the physiological scenario, which is most relevant to test pharmacological and non-pharmacological interventions to correct endothelial dysfunction, it is worth highlighting three animal models. The first are normolipidemic and normotensive Wistar rats which permit to analyze endothelial physiology in the absence of any additional cardiovascular risk factors. The second animal model are rats with congenital arterial hypertension (e.g., SHR rats). The third animal model are hyperlipidemic (ApoE or LDLR knockout) mice inherently prone to hyper/dyslipidemia, lipid deposition, and atherosclerosis.
The use of these models makes it possible to study endothelial dysfunction in the absence of modifiable cardiovascular risk factors, as well as in the two most common and important cardiovascular risk factors, arterial hypertension and hyper/dyslipidemia. Since these conditions are inevitably age-related processes rather than comorbidities, it is reasonable to consider these animal models as a kind of normal pathophysiological scenario for modeling endothelial dysfunction, in addition to the physiological scenario observed in Wistar rats.
The most suitable vessel for the analysis of endothelial dysfunction in rats and mice is the aorta, since: (1) this vessel belongs to the arterial bed (which makes it possible to associate endothelial dysfunction with the development of atherosclerosis, restenosis and aneurysms); (2) it contains both segments with atherogenic turbulent flow (aortic arch and bifurcation) and atheroprotective laminar flow (descending aorta); (3) has the maximum diameter among all other blood vessels, permitting to collect a sufficient amount of material for analysis. Albeit the descending aorta is the only possible choice as a segment with laminar flow due to its length and simplicity of its geometry, there are two options for choosing a segment with turbulent flow (as mentioned above, the aortic arch and bifurcation). Comparing these vascular segments, it is worth noting the extremely small size of the bifurcation fragment itself (its part with truly turbulent blood flow) even in comparison with the relatively small aortic arch. Therefore, it seems reasonable to prefer the aortic arch to analyze the endothelial physiology in turbulent blood flow.
Analysis of the endothelium in the aortic arch and descending aorta in situ can be performed in at least three ways: by rapidly washing the indicated aortic segments with Trizol in order to collect endothelial lysate for subsequent RNA isolation and profiling by RT-qPCR, by similar washing with RIPA buffer with a cocktail of protease and phosphatase inhibitors, or by using longitudinal dissection of the whole segment and its staining with primary and secondary fluorescently labeled antibodies, followed by en face imaging, which implies microscopy of the aorta laid intima up without any sectioning.
Collection of endothelial lysate with Trizol or RIPA buffer is advisable to be carried out using an insulin syringe after a thorough washing with saline sodium chloride solution to get rid of the blood. A single but slow syringe flush with Trizol or RIPA buffer is sufficient to isolate sufficient RNA or protein from rat aortic segments (although a fast thrice flushing is also justified). In order to achieve a protein concentration sufficient for Western blotting, the volume of RIPA buffer should not exceed 200 µL for the aortic arch and 300 µL for the descending aorta; it should probably be even smaller for mice, which have smaller aortic diameter. The histological justification for the use of this technique in rats and mice is that their intima normally consists exclusively a monolayer of endothelial cells, does not contain any other cell populations, and is separated from the medial layer containing vascular smooth muscle cells by an internal elastic lamina. Certainly this technique is associated with some degree of contamination by vascular smooth muscle cells (which is confirmed by the detection of alpha smooth muscle actin by Western blotting and the corresponding transcripts by RT-qPCR), however, it can be pre-optimized for each specific experiment, and it is advisable to make a correction for the amount of alpha smooth muscle actin or the corresponding transcripts in the control and experimental groups (by analogy with the ΔCt principle during analysis of RT-qPCR data).
The Western blotting peculiarity of rat proteins is that molecular weights of the detected proteins often do not match those in humans, which are used by antibody manufacturers as the reference. In addition to the fact that this peculiarity may raise questions for reviewers demanding technical details, it limits the simultaneous use of two or more primary antibodies, sometimes practiced in order to save resources in the analysis of human proteins in the case of preliminary testing of such antibodies, their divergence in molecular weights and the absence of their non-specific binding to non-target proteins or their fragments. However, these limitations do not diminish the technical validity of Western blotting in rat endothelial lysate.
Another analysis mentioned above is en face endothelial confocal microscopy. The need to use confocal but not epifluorescence microscopy is due to the strong background signal from the underlying internal elastic lamina in the green fluorescent channel, which requires cutting off individual optical sections to visualize endothelial cells stained with fluorescently labeled antibodies. A combination of Alexa Fluor 555- and Alexa Fluor 647-conjugated secondary antibodies that fluoresce in the red and far red channels should probably be used to minimize such autofluorescence. Although this method of analyzing the endothelial physiology is acceptable, its evidentiary power in most cases (excepting the assessment of the endothelial-mesenchymal transition) seems to be limited due to the complementary sense of the semi-quantitative analysis of confocal images; in addition, its use leads to a rather irrational use of the available tissue, since a sufficiently large part of the aortic segment is spent on staining with just one combination of antibodies.
MECHANISMS OF ENDOTHELIAL DYSFUNCTION
Pro-inflammatory activation
The main criteria for pro-inflammatory activation of the endothelium are adhesion of leukocytes to endothelial cells due to the binding of leukocyte receptors to the corresponding receptors of endothelial cells and an increased release of pro-inflammatory cytokines by endothelial cells [16–18]. Leukocyte receptors responsible for the attachment of leukocytes to endothelial cells include VLA-4/CD49d (binding to VCAM1 of endothelial cells), Mac-1 (complex of CD11b and CD18, binding to ICAM1 of endothelial cells), LFA-1/CD11a (binding to ICAM1 of endothelial cells) and PSGL-1/CD162 (association with E-selectin of endothelial cells) [19–22].
An objective analysis of leukocyte adhesion to the endothelium is impossible under static conditions due to a passive sedimentation and inevitable adhesion of monocytes and lymphocytes both to endothelial cells and to a culture plastic even after a number of washing stages (7−10). Even at complete confluence of the endothelial monolayer, it is impossible to separate the true adhesion of leukocytes to endothelial cells from the pseudoadhesion due to a mechanical attachment of monocytes and lymphocytes to the underlying surface, regardless of its coverage with cells. At the same time, the addition of leukocytes to the flow culture system in the amount of 1.25 × 105 cells per 1 mL (equivalent to 1.5 × 106 cells per module of the flow system) makes it possible to exclude such pseudoadhesion and to ensure a sufficient number of monocytes and lymphocytes adhered to the endothelial monolayer. This assay can be visualized using inverted fluorescence microscopy using any alternative nuclear stain (e.g., CellTracker Green) to label the leukocytes prior to their addition into the flow system and a standard nuclear stain (e.g., Hoechst 33342) for labeling cells inside the chamber after stopping the system and removing it immediately before imaging. In this design, leukocytes emit both blue and green fluorescence (using CellTracker Green), while endothelial cells give only blue fluorescence. For the better visualization, it is recommended to combine phase contrast and fluorescence imaging on an inverted microscope.
Although the analysis of leukocyte adhesion to endothelial cells is a functional analysis with a strong evidence for pro-inflammatory activation of the endothelium, obtaining several lines of evidence demands measurement of the expression of genes and proteins responsible for the adhesion of leukocytes to endothelial cells. These genes should include VCAM1, ICAM1, SELE, and SELP, which encode the vascular cell adhesion molecule, intercellular cell adhesion molecule, E-selectin, and P-selectin, respectively. The optimal method for measuring the expression of these genes is various types of polymerase chain reaction (e.g., RT-qPCR or digital droplet PCR), and Western blotting is a technique of choice for measuring protein expression. Intriguingly, VCAM1 and ICAM1 expression significantly exceeds that of E-selectin allowing them to be used as markers of pro-inflammatory activation). A semi-quantitative analysis of confocal microscopy images could be an alternative approach to measure the expression of these proteins yet numerous attempts to use this method in different experiments did not lead to satisfactory results. With regards to cell adhesion molecules, it is recommended to use confocal microscopy solely for the qualitative analysis of their localization (e.g., in the cytosol or on the plasma membrane), and not for the quantitative analysis of their expression. However, confocal microscopy visualization can complement RT-qPCR and Western blotting data. Thus, semi-quantitative analysis of confocal images is used either to study localization of an object of interest inside a cell, including its association with specific organelles or as a complementary but not independent analytical method.
With regard to the increased secretion of pro-inflammatory cytokines, endothelial cells release six: interleukin (IL)-6, IL-8, monocyte chemoattractant molecule 1 (MCP-1 / CCL2), CXCL1 (growth-associated oncogene, GRO-α), macrophage migration inhibitory factor (MIF), and plasminogen activator inhibitor (PAI-1, serpin E1) [23, 24]. The range of pro- and anti-angiogenic molecules secreted by endothelial cells is much wider [23, 24], while pro-angiogenic molecules include angiopoietin-1 [25, 26], a soluble form of the vascular endothelial growth factor receptor (VEGFR2) [27, 28], angiogenin [29, 30], epidermal growth factor (EGF) [31, 32], placental growth factor (PlGF) [33, 34], platelet growth factor (more precisely, its subunits PDGF-AA and PDGF-BB) [35, 36], basic fibroblast growth factor (bFGF) [37, 38], endothelin-1 [39, 40], pentraxin 3 [41, 42], dipeptidyl peptidase 4 [43, 44], CD105/endoglin [45, 46], hepatocyte growth factor (HGF) [47, 48], insulin growth factor-binding proteins IGFBP-1 [49, 50], IGFBP-2 [51, 52] and IGFBP-3 [53, 54], CXCL16 [55, 56], transforming growth factor (TGF-b1) [57, 58], urokinase plasminogen activator (uPA) and corresponding soluble receptor (uPAR) [59, 60], matrix metalloproteinase-1 (MMP-1) [60, 61], IL-6 [62, 63], IL-8 [64, 65], and MCP-1/CCL2 [66, 67], while anti-angiogenic molecules include angiopoietin-2 [68, 69], thrombospondin-1 [70, 71], endostatin [72, 73], and tissue inhibitors of metalloproteinases (TIMP-1 [74, 75], TIMP-2 [76, 77]) and a plasminogen activator inhibitor (PAI-1)) [78, 79]. Measurement of cytokines, pro- and anti-angiogenic factors in a culture medium from endothelial cells is performed primarily through semi-quantitative profiling using dot blotting, as specialized kits available on the market allow the determination of all abovementioned cytokines and the vast majority of pro- and anti-angiogenic proteins. Such screening permits to reveal the secreted molecules, which are differentially expressed between the experimental and control groups, and then conduct a targeted quantitative analysis using high-sensitive enzyme-linked immunosorbent assay. If it is not possible to perform a dot blot (e.g., due to the lack of a chemiluminescent scanner), measurement of IL-6, IL-8 and MCP-1/CCL2 levels in the culture medium by enzyme-linked immunosorbent assay also permits to estimate the pro-inflammatory activation of endothelial cells. Prior to dot blotting or enzyme immunoassay, low speed centrifugation of the collected medium (1,000−3,000 g) should be performed to remove cellular debris.
In addition to the measurement of pro-inflammatory cytokines released by endothelial cells into the microenvironment, it is also recommended to conduct the experiments with conditioned serum-free medium, which should be added to intact cultures of endothelial cells within 24 h of conditioning for no more than 24 h to evaluate its pathological effects. Fractionation of the conditioned medium into extracellular vesicles and vesicle-free medium by ultracentrifugation might also be useful. Before transferring the conditioned medium from dysfunctional to intact endothelial cells, it should be subjected to low-speed centrifugation in order to remove cellular debris and associated molecules.
The studies by our group have shown that the addition of calciprotein particles into the flow system leads to their internalization by endothelial cells within 1 hour and provokes death of a small but statistically significant proportion (1−2%) of endothelial cells within 4 hours due to a pathological permeabilization of lysosomes and mitochondria and stimulates the release of IL-6, IL-8 and MCP-1/CCL2 [80, 81]. This made it possible to consider calciprotein particles as a model trigger of endothelial dysfunction.
Subsequent experiments showed that a 4-h incubation of primary coronary artery and internal thoracic artery endothelial cells with calciprotein particles causes adhesion of leukocytes to arterial endothelial cells under flow accompanied by an increase in VCAM1 and ICAM1 [82–84]. Both complete and vesicle-free conditioned medium from CPP-treated primary coronary artery endothelial cells led to an increased expression of VCAM1, ICAM1, SELE and SELP genes in combination with an elevated expression of IL6, CXCL8, CCL2, CXCL1 and MIF genes, increased expression of VCAM1 and ICAM1 receptors, and augmented release of IL-6, IL-8 and MCP-1/CCL2 when added to intact human coronary artery endothelial cells. Yet, the addition of extracellular vesicles from CPP-treated endothelial cells did not lead to such effects, indicating the leading role of the soluble factors in the development of paracrine pro-inflammatory endothelial activation. Since no shift of pro- and anti-angiogenic factors secreted by endothelial cells into the environment was detected, it was assumed that pro-inflammatory cytokines (IL-6, IL-8, MCP-1/CCL2) play the main role in this pathophysiological scenario.
Thus, pro-inflammatory activation should be considered as the first and foremost feature of endothelial dysfunction and is defined as: (1) increased adhesion of leukocytes to endothelial cells during their co-incubation under flow, accompanied by an increase in the expression of VCAM1 and ICAM1 proteins and the corresponding genes; (2) increased release of IL-6, IL-8 and MCP-1/CCL2 into the microenvironment; (3) pathological paracrine effects of endothelial cells (e.g., the ability of the conditioned medium to cause endothelial dysfunction in intact endothelial cell cultures).
Impairment of endothelial mechanotransduction
Another aspect of endothelial pathophysiology that cannot be studied under static culture conditions is disturbed endothelial mechanotransduction (transformation of mechanical cues, e.g., flow, into the biochemical signals). Mechanotransduction plays a pivotal role in endothelial physiology in vivo, as reflected by the susceptibility of vascular segments with turbulent blood flow (e.g., the aortic arch) to atherosclerosis compared to vascular segments with laminar blood flow (e.g., the descending aorta) [16–18]. In addition to a variety of mechanosensors including VEGFR2 [85, 86], Tie2 [87], and PECAM-1/CD31 [88, 89], integrins and associated adhesion foci [90–93], intercellular contact proteins VE-cadherin and occludin [85, 89, 94], stretch-activated ion channels [95, 96], transmembrane proteoglycans [97], G-protein-coupled receptors [98], heterotrimeric G-proteins [99, 100], glycocalyx [101, 102], caveolin-1 and caveolae [103, 104], primary cilia [105, 106], intermediate filaments [107, 108], and actin cytoskeleton [109–111], signal transmission from mechanosensors occurs through a number of specialized transcription factors: KLF2 [112–114], KLF4 [115–117], NRF2 [118, 119], YAP and TAZ [120, 121]. Three of these factors (KLF2, KLF4, and NRF2) are atheroprotective [113–116, 118, 119, 122–131], while two (YAP and TAZ) are atherogenic [120, 121, 131–134].
The function of the atherogenic transcription factors YAP and TAZ is largely regulated at the post-translational level through phosphorylation (which provokes their sequestration in the cytosol and degradation of these proteins through ubiquitination in proteasomes), which reflects the need to evaluate not only the total but also the phosphorylated fraction of these molecules. Another way to analyze the activity of atherogenic transcription factors YAP and TAZ is the comparative assessment of total and phosphorylated forms of these proteins in the cytosol and nucleus by Western blotting after cell fractionation. Unfortunately, this technique requires a significant cell amount that is impossible to be obtained in flow culture chambers, yet static conditions does not permit analysis of endothelial mechanotransduction. Attempts to assess the fluorescence intensity of total and phosphorylated forms of YAP and TAZ in the cytosol and nucleus during immunostaining also did not lead to any unbiased result.
While the absence of external biomechanical cues makes impossible proper modeling of endothelial mechanotransduction, culturing in a flow system meets several conditions for this task: constant exposure to unidirectional pulsating flow with controlled shear stress, elongation of endothelial cells with the flow, and ensuring cell-cell contacts between endothelial cells within the monolayer. Internalization of CPPs led to a decrease in the expression of mechanosensitive atheroprotective transcription factors KLF2 and KLF4, also reducing the phosphorylation of the atherogenic transcription factor YAP1 and thus leading to its activation, which is suppressed under physiological conditions. At the same time, these effects were observed both in primary endothelial cell cultures and in the endothelium of rat descending aorta and aortic arch. These alterations were particularly pronounced in the aortic arch characterized by turbulent blood flow, where the Yap1 protein was hypophosphorylated at both residues (Ser127 and Ser397) and the Taz protein was also hypophosphorylated, reflecting the resistance of these two atherogenic transcription factors to degradation. Thus, both cultures of endothelial cells incubated with CPPs and aortic endothelium of rats subjected to intravenous administration of CPPs had molecular imprints of impaired mechanotransduction.
When attempting to formulate criteria for endothelial dysfunction, one should mention the absence of any functional analysis (similar to the analysis of leukocyte adhesion to endothelial cells) or any biomarker released into the microenvironment to draw an unambiguous conclusion on impaired endothelial mechanotransduction. However, its signs may include reduced expression of atheroprotective transcription factors KLF2, KLF4, and NRF2 and their encoding genes (KLF2, KLF4, NFE2L2), as well as hypophosphorylation of atherogenic transcription factors YAP1 and TAZ that leads to their activation. Which of these changes are the most pathophysiologically significant is debatable; therefore, it seems reasonable to define disturbed endothelial mechanotransduction as a combination of decreased expression of atheroprotective transcription factors and activating hypophosphorylation of atherogenic transcription factors. In accordance with this definition, impaired endothelial mechanotransduction can be designated as one of the main endothelial dysfunction features.
Endothelial-to-mesenchymal transition
An equally important aspect of endothelial dysfunction is the endothelial-to-mesenchymal transition that accompanies the development of atherosclerosis and contributes to the loss of endothelial cell orientation, disintegration of intercellular contacts, increased expression of cell adhesion molecules, and switch to a synthetic phenotype [135–139]. The endothelial-mesenchymal transition is accompanied by a gradual decrease in the expression of endothelial markers (PECAM-1/CD31, VE-cadherin/CD144, VEGFR2/KDR/CD309) and an increase in the expression of mesenchymal markers (N-cadherin/CD325, alpha-smooth muscle actin, vimentin, calponin, SM22α, fibroblast-associated protein, fibroblast-specific protein) and is regulated by the transcription factors Snail, Slug, Twist1, Gata4, and Zeb1 [137, 139–141]. Expression of the transcription factor Gata4 in human endothelial cells is questionable, while the other four transcription factors are in significant amounts both at the transcript and protein level.
Among these, the most important transcription factors are Snail and Slug, two cognate transcription factors, which can be simultaneously detected by Western blotting with one of the commercially available antibodies recognizing both of them. However, this antibody is polyclonal, while current Western blotting specificity requirements strongly recommend the use of monoclonal antibodies to minimize their non-specific binding. However, the difference in the molecular weights of the endothelial-to-mesenchymal transition transcription factors makes it possible to concurrently evaluate the expression of Snail/Slug (or Twist1), which are generally localized at molecular weights up to 50 kDa, and the transcription factor Zeb1, which can be determined on molecular weight of 200 kDa.
In addition to Western blotting, an objective assessment of endothelial-to-mesenchymal transition can also be carried out using the gene expression analysis, since the activity of transcription factors or markers of this process does not depend on post-translational modifications. In this case, a changed expression of the respective transcription factor genes, namely SNAI1, SNAI2, TWIST1 and ZEB1 is observed, although the differential expression of each of them significantly depends on the type of vascular endothelium (cell line in vitro or vascular segment in vivo). Changes in the expression of the endothelial-to-mesenchymal transition markers occur much later and serve as a signal of ongoing transdifferentiation. The most convincing evidence of such transdifferentiation is probably immunofluorescence staining, which indicates the acquisition of mesenchymal markers by endothelial cells. A classic example of this phenomenon is combined staining for the endothelial cell marker PECAM-1/CD31 and the vascular smooth muscle cell marker alpha smooth muscle actin, as well as for another endothelial cell marker VE-cadherin/CD144 and the mesenchymal cell marker N-cadherin/CD325. The relevance of immunofluorescence staining to determine the endothelial-to-mesenchymal transition probably also makes it possible to prove its implementation in vivo when dissecting an entire aortic segment, staining it with primary and secondary fluorescent-labeled antibodies, and subsequent en face imaging. This method allows distinguishing cells with an endothelial phenotype (CD31+αSMA− or CD144+CD325−) from cells with a mixed (CD31+αSMA+ or CD144+CD325+) or mesenchymal (CD31−αSMA+ or CD144−CD325+) phenotype and provides a technical opportunity to count the cells with a transitional or mesenchymal phenotype. With regard to the secretome of endothelial cells undergoing an endothelial-to-mesenchymal transition (considering this process as a continuum but not discrete transition between two states), a practical assessment of the soluble factors released into the microenvironment during the endothelial-to-mesenchymal transition is quite difficult because of its duration (1−2 weeks).
Prolonged intravenous administration of CPPs to Wistar rats initiated the endothelial-mesenchymal transition by increasing the expression of its transcription factors Snail, Slug and Twist1 in the endothelium of descending aorta and aortic arch. Trends in gene and protein expression suggested that the endothelial-to-mesenchymal transition develops over time along with the CPP exposure, and the increased expression of its transcription factor genes (SNAI1, SNAI2 and ZEB1) after the internalization of CPPs precedes the subsequent overexpression of the proteins Snail, Slug and Twist1 regulating change of endothelial to mesenchymal phenotype.
Thus, the criteria for endothelial-to-mesenchymal transition include: (1) the acquisition of mesenchymal phenotype markers (primarily N-cadherin and alpha-smooth muscle actin); (2) increased expression of at least three out of four corresponding transcription factors (Snail, Slug, Twist1, and Zeb1). The endothelial-to-mesenchymal transition itself should also be defined as a continuum of events but not as an accomplished fact of the mesenchymal phenotype acquisition. Since the endothelial-to-mesenchymal transition is associated with both pro-inflammatory endothelial activation (leukocyte adhesion to endothelial cells and release of pro-inflammatory cytokines [83, 142, 143]) and is induced by chronic inflammation [144, 145], turbulent flow [135] and impaired endothelial mechanotransduction [146, 147] endothelial-to-mesenchymal transition can also be considered as a mandatory feature of endothelial dysfunction.
Impaired biosynthesis and release of nitric oxide (NO)
One of the key aspects of endothelial dysfunction is the reduced ability of endothelial cells to produce nitric oxide (NO), which is a key vasodilator. From a methodological point of view, four stages can be distinguished at which an indirect assessment of NO release is possible: transcription of the gene for the key enzyme of this process, endothelial NO synthase (NOS3), synthesis of this protein in endothelial cells as a result of translation on ribosomes, functionally significant post-translational modifications of this enzyme (phosphorylation at the threonine-495 position that inhibits the activity of this enzyme and phosphorylation at serine-113 and serine-1177 positions increasing its activity), and the actual synthesis of NO reflected by the amount of nitrates (NO3) and nitrites (NO2) since the NO molecule itself exists for only a few seconds after its biosynthesis. In addition to assessing the components of the NO synthesis chain, when assessing endothelial dysfunction it is also important to analyze the functional activity of reactive nitrogen species, in particular, peroxynitrite (ONOO−), which is formed when the endothelial NO synthase signaling pathway is uncoupled as soon as this enzyme switches on the synthesis of superoxide (O2 •−) instead of NO. Peroxynitrite (ONOO−) in this case is formed from the superoxide generated by the uncoupling of the endothelial NO synthase signaling pathway and the synthesized free NO. Other reactive nitrogen species are NO2 and N2O3, formed by the reactions of peroxynitrite (ONOO−) with other chemical compounds. All reactive nitrogen species also have a very short lifespan, and 3-nitrotyrosine, a reliable marker of nitrosative stress, can be detected by Western blotting with the appropriate antibody. In addition, since nitrosative stress is linked to oxidative stress through the excessive superoxide synthesis during NO synthase signaling pathway uncoupling, it is also advisable to analyze the functional activity of reactive oxygen species by measuring the levels of thiobarbituric acid reaction products (TBARS) which include malondialdehyde, 8-isoprostane, and 4-hydroxynonenal and are products of free radical metabolism (primarily superoxide O2 •−, hydrogen peroxide H2O2, hydroxyl radical •OH, and singlet oxygen 1[O2]).
When measuring transcription of the NOS3 gene in humans, it is important to keep in mind the four isoforms of this enzyme that must be detected using a single primer. When evaluating post-translational modifications of this protein, it is of fundamental importance to compare the relative amount of its total form, as well as inhibitory and activating phosphorylated forms. In contrast to the transcription factors of endothelial mechanotransduction YAP and TAZ, which phosphorylated forms are relatively easily detected in endothelial cell lysate, detection of phosphorylated forms of NO synthase in cell culture lysate in vitro (unlike in vivo endothelial lysate) is quite difficult and requires a large amount of loading protein for Western blotting in combination with a small dilution of the corresponding primary antibodies. Measurement of nitrates and nitrites as metabolic products of NO released by endothelial cells in the culture medium, as well as measurement of thiobarbituric acid reaction products as metabolic products of reactive oxygen species, can be carried out by colorimetric analysis using appropriate kits. Since the detection of nitrates, nitrites, and thiobarbituric acid reaction products in the culture medium also requires their concentration above the detectable limit, it is recommended to carry out such experiments under static conditions with a serum-free culture medium to prevent contamination with nitrates, nitrites and reactive oxygen species from the serum after at least 24 h of endothelial cell incubation and to perform the measurements after low-speed centrifugation to get rid of the cell debris.
The addition of CPPs to endothelial cell cultures into the pulsatile flow system caused a decrease in the expression of endothelial NO synthase and the corresponding NOS3 gene. It was also found that the endothelium of normolipidemic and normotensive Wistar rats which underwent regular intravenous administration of CPPs was characterized by an increased expression of the phosphorylated endothelial NO synthase at the Thr495 position as well as elevated 3-nitrotyrosine in the descending aorta. These results indicated disturbed functioning of endothelial NO synthase as a result of CPP internalization and confirmed the validity of their use for modeling endothelial dysfunction.
Detection of impaired NO biosynthesis and release is quite difficult due to its complexity (e.g., measuring the level of nitrates, nitrites and thiobarbituric acid reaction products in vivo will not reflect endothelial dysfunction in any particular vessel), while the absence of pathological changes in any of the NO biosynthesis components (e.g., inhibitory post-translational modifications) does not necessarily indicate the absence of a decrease in NO release by endothelial cells. Additional complexity is provided by the NO synthase signaling pathway uncoupling, in which even with a normal level and functioning of this enzyme, the release of NO by endothelial cells is reduced and is accompanied by nitrosative and oxidative stress. The most reasonable option is that the disruption of NO biosynthesis is determined both by the detectable decrease in NO in the endothelial cell microenvironment and by a decrease in the expression of the NOS3 gene and the endothelial NO synthase, as well as an increase in the ratio of its Thr495 phosphorylated form to the total fraction of this enzyme. Measurements of 3-nitrotyrosine level and thiobarbituric acid reaction products represent a good complementary option. As mentioned above, disruption of NO biosynthesis and NO release is an essential component of endothelial dysfunction.
Loss of endothelial integrity
The final component of endothelial dysfunction proposed for consideration is a pathological increase in the endothelial permeability, resulting in in vivo penetration of atherogenic lipoproteins, cytokines and immune cells into the intima. Experimentation under flow is a mandatory prerequisite for modeling this pathological process, which is most closely related to the cerebral endothelium (due to the importance of ensuring the semi-permeability of the blood–brain barrier for the integrity of the central nervous system tissues).
Pathological increase in endothelial permeability can be modeled by adding plasma from diseases patients (e.g., those with septic shock) or culture medium with an endothelial dysfunction trigger to a pulsatile flow system connected to an endothelial cell-populated culture flow camera optionally coated with type IV collagen to mimic the basement membrane. After the required incubation time, endothelial cells inside the control and experimental chambers can be stained with antibodies to VE-cadherin (a marker of intercellular contacts staining for which makes it possible to assess the integrity of the endothelial monolayer) and F-actin (a marker of cytoskeleton orientation, which can be used to judge physiological and pathological changes in cell geometry). Correspondingly, violated integrity of the endothelium during confocal microscopy imaging will be defined as the loss of intercellular contacts (by staining with antibodies to VE-cadherin) and the loss of endothelial cell elongation (by staining by antibodies to F-actin). Automated analysis of changes in cellular geometry can be carried out using the Directionality and OrientationJ plugins in the ImageJ program.
The classic method for assessing the disrupted endothelial integrity in vivo is staining with Evans Blue, a dye that binds to albumin, a high molecular weight protein that normally does not penetrate tissues through the intact endothelium. If the integrity of the endothelium is compromised, albumin can penetrate into the intima (or through the blood–brain barrier) and binds to proteins and proteoglycans of the intercellular matrix, which is reflected by blue staining of the vessels after their excision (normally, they retain a native color since Evans blue-stained albumin is not able to penetrate through intact endothelium).
Despite the obvious pathophysiological effects of impaired endothelial integrity, it is a consequence rather than a mandatory component of endothelial dysfunction. For example, when modeling endothelial dysfunction by adding CPPs to a pulsating flow system or their intravenous administration to laboratory animals, it was shown that despite their certain toxicity (statistically significant death of 1−2% endothelial cells) and the ability to cause all abovementioned features of endothelial dysfunction (pro-inflammatory activation, impaired endothelial mechanotransduction, endothelial-to-mesenchymal transition, and impaired NO biosynthesis), the severity of these pathological effects in this scenario is insufficient to disrupt the integrity of the endothelial monolayer in the culture flow chambers. In addition, in spite of its ability to induce the formation of neointima and adventitial/perivascular inflammation, regular intravenous administration of CPPs also did not lead to the disturbed endothelial integrity according to the results of Evans blue staining.
Prothrombotic activation of the endothelium
The importance of prothrombotic activation of the endothelium, which is especially important in the context of COVID-19 [148–150], should also be noted. The main markers of prothrombotic activation are pathologically increased release of von Willebrand factor and plasminogen activator inhibitor (PAI-1) in combination with a reduced release of various plasminogen activators (uPA and tPA). Prothrombotic endothelial activation definitely must be attributed to the endothelial dysfunction features, however, due to the lack of such experiments in the authors’ practice, we do not go into a more detailed discussion in this article.
CONCLUSIONs
When speaking about the definition and criteria of endothelial dysfunction, it seems important to emphasize the versatility of this phenomenon, which affects not only NO biosynthesis, but covers almost all aspects of endothelial physiology. In particular, the criteria for endothelial dysfunction are:
1. Pro-inflammatory activation, determined by pronounced adhesion of leukocytes to endothelial cells (accompanied by an increase in the expression of VCAM1 and ICAM1 proteins and their encoding genes), increased release of the main endothelial pro-inflammatory cytokines (IL-6, IL-8 and MCP-1/CCL2) and pathogenic paracrine effects of dysfunctional endothelial cells in the experiment.
2. Impaired endothelial mechanotransduction, defined as a combination of decreased expression of atheroprotective transcription factors (KLF2, KLF4 and NRF2) and activating hypophosphorylation of atherogenic transcription factors (YAP and TAZ).
3. Endothelial-to-mesenchymal transition, defined as a continued acquisition of the mesenchymal markers (primarily N-cadherin and alpha smooth muscle actin) against the increased expression of at least three out of four relevant transcription factors (Snail, Slug, Twist1 and Zeb1).
4. Disturbed NO biosynthesis, determined both by the actual decrease in NO metabolic products in the endothelial cell microenvironment and by a reduced NOS3 gene and endothelial NO synthase expression, as well as by increased ratio of its phosphorylated Thr495 form to the total eNOS.
5. Prothrombotic activation of the endothelium, reflected by a pathologically increased release of von Willebrand factor and plasminogen activator inhibitor (PAI-1) in combination with a reduced secretion of various plasminogen activators (uPA and tPA).
6. Additional criterion, absolutely confirming the development of endothelial dysfunction, is its impaired integrity characterized by a pathological increase in endothelial permeability.
Thus, endothelial dysfunction should be defined as a complex pathological changes in its physiology, including pathophysiologically significant pro-inflammatory and prothrombotic activation of endothelial cells, impaired endothelial mechanotransduction, endothelial-to-mesenchymal transition, and compromised NO biosynthesis. With regard to systemic biomarkers of endothelial dysfunction in vivo, it should be noted that so far no single reliable biomarker has been identified. Evidence-based and sensitive markers of endothelial dysfunction in vitro are IL-6, IL-8, and MCP-1/CCL2, however, these pro-inflammatory cytokines are also produced by other cell populations (e.g., leukocytes) and therefore are not specific to the in vivo scenario.
It is recommended to use VCAM1, ICAM1, SELE and SELP (endothelial cell receptors for leukocytes), IL6, CXCL8, CCL2, CXCL1 and MIF (major endothelial pro-inflammatory cytokines), KLF2, KLF4 and NFE2L2 (mechanosensitive atheroprotective transcription factors), SNAI1, SNAI2, TWIST1, ZEB1, CDH5 and CDH2 (transcription factors and markers of the endothelial-to-mesenchymal transition), NOS3 (endothelial NO synthase gene), VWF, SERPINE1, PLAU and PLAT genes (factors responsible for endothelium-dependent hemostasis), as well as their corresponding proteins to determine an endothelial dysfunction (Table 1). In this experimental design, increased expression of the VCAM1, ICAM1, SELE, and SELP genes, combined with the elevated expression of the IL6, CXCL8, CCL2, CXCL1 and MIF genes, is indicative of pro-inflammatory endothelial cell activation; decreased expression of the KLF2, KLF4, and NFE2L2 genes (under flow conditions) is an indicator of impaired mechanotransduction, increased expression of the SNAI1, SNAI2, TWIST1 and ZEB1 genes in combination with reduced expression of the VE-cadherin gene CDH5 and increased expression of the N-cadherin gene CDH2 indicates an endothelial-mesenchymal transition, reduced expression of the NOS3 gene suggests an impaired ability of endothelial cells to secrete NO, and increased expression of the VWF and SERPINE1 genes in combination with reduced expression of the PLAU and PLAT genes is an indicator of prothrombotic activation of the endothelium. A similar statement is also true for the proteins encoded by these genes, however, preliminary screening of these genes using RT-qPCR seems to be the optimal approach due to the relatively low cost and technical complexity of this method. If the above signs of endothelial dysfunction are detected, they can be verified using Western blotting or enzyme-linked immunosorbent assay (in the case of pro-inflammatory cytokines) or extended profiling by whole transcriptome sequencing or liquid chromatography-mass spectrometry (Table 2).
REFERENCES
Scioli MG, Storti G, D’Amico F, Rodríguez Guzmán R, Centofanti F, Doldo E, Céspedes Miranda EM, Orlandi A (2020) Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J Clin Med 9(6):1995. https://doi.org/10.3390/jcm9061995
Liao JK (2013) Linking endothelial dysfunction with endothelial cell activation. J Clin Invest 123(2):540–541. https://doi.org/10.1172/JCI66843
Mauricio MD, Aldasoro M, Ortega J, Vila JM (2013) Endothelial dysfunction in morbid obesity. Curr Pharm Des 19(32):5718–5729. https://doi.org/10.2174/1381612811319320007
Pomilio M, Mohn A, Verrotti A, Chiarelli F (2002) Endothelial dysfunction in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 15(4):343–361. https://doi.org/10.1515/jpem.2002.15.4.343
Totoson P, Maguin-Gaté K, Prati C, Wendling D, Demougeot C (2014) Mechanisms of endothelial dysfunction in rheumatoid arthritis: lessons from animal studies. Arthritis Res Ther 16(1):202. https://doi.org/10.1186/ar4450
Ferroni P, Basili S, Paoletti V, Davì G (2006) Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr Metab Cardiovasc Dis 16(3):222–233. https://doi.org/10.1016/j.numecd.2005.11.012
Prati C, Demougeot C, Guillot X, Godfrin-Valnet M, Wendling D (2014) Endothelial dysfunction in joint disease. Joint Bone Spine 81(5):386–391. https://doi.org/10.1016/j.jbspin.2014.01.014
Simonsen U, Rodriguez-Rodriguez R, Dalsgaard T, Buus NH, Stankevicius E (2009) Novel approaches to improving endothelium-dependent nitric oxide-mediated vasodilatation. Pharmacol Rep 61(1):105–115. https://doi.org/10.1016/s1734-1140(09)70012-x
Bertani F, Di Francesco D, Corrado MD, Talmon M, Fresu LG, Boccafoschi F (2021) Paracrine Shear-Stress-Dependent Signaling from Endothelial Cells Affects Downstream Endothelial Function and Inflammation. Int J Mol Sci 22(24):13300. https://doi.org/10.3390/ijms222413300
Ding H, Triggle CR (2010) Endothelial dysfunction in diabetes: multiple targets for treatment. Pflugers Arch 459(6):977–994. https://doi.org/10.1007/s00424-010-0807-3
O’Riordan E, Chen J, Brodsky SV, Smirnova I, Li H, Goligorsky MS (2005) Endothelial cell dysfunction: the syndrome in making. Kidney Int 67(5):1654–1658. https://doi.org/10.1111/j.1523-1755.2005.00256.x
Goligorsky MS (2005) Endothelial cell dysfunction: can’t live with it, how to live without it. Am J Physiol Renal Physiol 288(5):F871–F880. https://doi.org/10.1152/ajprenal.00333.2004
Pierce RW, Giuliano JS, Whitney JE, Ouellette Y; Pediatric Organ Dysfunction Information Update Mandate (PODIUM) Collaborative (2022) Endothelial Dysfunction Criteria in Critically Ill Children: The PODIUM Consensus Conference. Pediatrics 149 (1 Suppl 1):S97–S102. https://doi.org/10.1542/peds.2021-052888O
Soerensen M, Debrabant B, Halekoh U, Møller JE, Hassager C, Frydland M, Hjelmborg J, Beck HC, Rasmussen LM (2021) Does diabetes modify the effect of heparin on plasma proteins?—A proteomic search for plasma protein biomarkers for diabetes-related endothelial dysfunction. J Diabetes Complications 35(6):107906. https://doi.org/10.1016/j.jdiacomp.2021.107906
Le Brocq M, Leslie SJ, Milliken P, Megson IL (2008) Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal 10(9):1631–1674. https://doi.org/10.1089/ars.2007.2013
Cahill PA, Redmond EM (2016) Vascular endothelium—Gatekeeper of vessel health. Atherosclerosis 248:97–109. https://doi.org/10.1016/j.atherosclerosis.2016.03.007
Jensen HA, Mehta JL (2016) Endothelial cell dysfunction as a novel therapeutic target in atherosclerosis. Expert Rev Cardiovasc Ther 14(9):1021–1033. https://doi.org/10.1080/14779072.2016.1207527
Gimbrone MA Jr, García-Cardeña G (2016) Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 118(4):620–636. https://doi.org/10.1161/CIRCRESAHA.115.306301
Hyun YM, Lefort CT, Kim M (2009) Leukocyte integrins and their ligand interactions. Immunol Res 45(2-3):195–208. https://doi.org/10.1007/s12026-009-8101-1
McEver RP (2015) Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res 107(3):331–339. https://doi.org/10.1093/cvr/cvv154
Allen S, Moran N (2015) Cell Adhesion Molecules: Therapeutic Targets for Inhibition of Inflammatory States. Semin Thromb Hemost 41(6):563–571. https://doi.org/10.1055/s-0035-1556588
Dustin ML (2019) Integrins and Their Role in Immune Cell Adhesion. Cell 177(3):499–501. https://doi.org/10.1016/j.cell.2019.03.038
Shishkova D, Markova V, Sinitsky M, Tsepokina A, Frolov A, Zagorodnikov N, Bogdanov L, Kutikhin A (2020) Co-Culture of Primary Human Coronary Artery and Internal Thoracic Artery Endothelial Cells Results in Mutually Beneficial Paracrine Interactions. Int J Mol Sci 21(21):8032. https://doi.org/10.3390/ijms21218032
Kutikhin AG, Tupikin AE, Matveeva VG, Shishkova DK, Antonova LV, Kabilov MR, Velikanova EA (2020) Human Peripheral Blood-Derived Endothelial Colony-Forming Cells Are Highly Similar to Mature Vascular Endothelial Cells yet Demonstrate a Transitional Transcriptomic Signature. Cells 9(4):876. https://doi.org/10.3390/cells9040876
Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87(7):1171–1180. https://doi.org/10.1016/s0092-8674(00)81813-9
Lee J, Kim KE, Choi DK, Jang JY, Jung JJ, Kiyonari H, Shioi G, Chang W, Suda T, Mochizuki N, Nakaoka Y, Komuro I, Yoo OJ, Koh GY (2013) Angiopoietin-1 guides directional angiogenesis through integrin alphavbeta5 signaling for recovery of ischemic retinopathy. Sci Transl Med 5(203):203ra127. https://doi.org/10.1126/scitranslmed.3006666
Rezzola S, Di Somma M, Corsini M, Leali D, Ravelli C, Polli VAB, Grillo E, Presta M, Mitola S (2019) VEGFR2 activation mediates the pro-angiogenic activity of BMP4. Angiogenesis 22(4):521–533. https://doi.org/10.1007/s10456-019-09676-y
Zarkada G, Heinolainen K, Makinen T, Kubota Y, Alitalo K (2015) VEGFR3 does not sustain retinal angiogenesis without VEGFR2. Proc Natl Acad Sci U S A 112(3):761–766. https://doi.org/10.1073/pnas.1423278112
Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF (2005) Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene 24(3):445–456. https://doi.org/10.1038/sj.onc.1208223
He T, Qi F, Jia L, Wang S, Wang C, Song N, Fu Y, Li L, Luo Y (2015) Tumor cell-secreted angiogenin induces angiogenic activity of endothelial cells by suppressing miR-542-3p. Cancer Lett 368(1):115–125. https://doi.org/10.1016/j.canlet.2015.07.036
Karl E, Zhang Z, Dong Z, Neiva KG, Soengas MS, Koch AE, Polverini PJ, Núñez G, Nör JE (2007) Unidirectional crosstalk between Bcl-xL and Bcl-2 enhances the angiogenic phenotype of endothelial cells. Cell Death Differ 14(9):1657–1666. https://doi.org/10.1038/sj.cdd.4402174
Falcon BL, Swearingen M, Gough WH, Lee L, Foreman R, Uhlik M, Hanson JC, Lee JA, McClure DB, Chintharlapalli S (2014) An in vitro cord formation assay identifies unique vascular phenotypes associated with angiogenic growth factors. PLoS One 9(9):e106901. https://doi.org/10.1371/journal.pone.0106901
Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P, Young PP (2006) VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J 20(9):1495–1497. https://doi.org/10.1096/fj.05-5137fje
Gigante B, Morlino G, Gentile MT, Persico MG, De Falco S (2006) Plgf-/-eNos-/- mice show defective angiogenesis associated with increased oxidative stress in response to tissue ischemia. FASEB J 20(7):970–972. https://doi.org/10.1096/fj.05-4481fje
Shikada Y, Yonemitsu Y, Koga T, Onimaru M, Nakano T, Okano S, Sata S, Nakagawa K, Yoshino I, Maehara Y, Sueishi K (2005) Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Res 65(16):7241–7248. https://doi.org/10.1158/0008-5472.CAN-04-4171
Wu LW, Chen WL, Huang SM, Chan JY (2019) Platelet-derived growth factor-AA is a substantial factor in the ability of adipose-derived stem cells and endothelial progenitor cells to enhance wound healing. FASEB J 33(2):2388–2395. https://doi.org/10.1096/fj.201800658R
Esser JS, Rahner S, Deckler M, Bode C, Patterson C, Moser M (2015) Fibroblast growth factor signaling pathway in endothelial cells is activated by BMPER to promote angiogenesis. Arterioscler Thromb Vasc Biol 35(2):358–367. https://doi.org/10.1161/ATVBAHA.114.304345
Litwin M, Radwańska A, Paprocka M, Kieda C, Dobosz T, Witkiewicz W, Baczyńska D (2015) The role of FGF2 in migration and tubulogenesis of endothelial progenitor cells in relation to pro-angiogenic growth factor production. Mol Cell Biochem 410(1–2):131–142. https://doi.org/10.1007/s11010-015-2545-5
Salani D, Taraboletti G, Rosanò L, Di Castro V, Borsotti P, Giavazzi R, Bagnato A (2000) Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol 157(5):1703–1711. https://doi.org/10.1016/S0002-9440(10)64807-9
Spinella F, Rosanò L, Di Castro V, Natali PG, Bagnato A (2002) Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J Biol Chem 277(31):27850–27855. https://doi.org/10.1074/jbc.M202421200
Rodriguez-Grande B, Varghese L, Molina-Holgado F, Rajkovic O, Garlanda C, Denes A, Pinteaux E (2015) Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia. J Neuroinflammation 12:15. https://doi.org/10.1186/s12974-014-0227-y
Rajkovic I, Wong R, Lemarchand E, Rivers-Auty J, Rajkovic O, Garlanda C, Allan SM, Pinteaux E (2018) Pentraxin 3 promotes long-term cerebral blood flow recovery, angiogenesis, and neuronal survival after stroke. J Mol Med (Berl) 96(12):1319–1332. https://doi.org/10.1007/s00109-018-1698-6
Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, Yeh Y, Chen WT, Kleinman HK, Grouzmann E, Grant DS (1998) Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res 83(2):187–195. https://doi.org/10.1161/01.res.83.2.187
Ghersi G, Chen W, Lee EW, Zukowska Z (2001) Critical role of dipeptidyl peptidase IV in neuropeptide Y-mediated endothelial cell migration in response to wounding. Peptides. 22(3):453–458. https://doi.org/10.1016/s0196-9781(01)00340-0
Duff SE, Li C, Garland JM, Kumar S (2003) CD105 is important for angiogenesis: evidence and potential applications. FASEB J 17(9):984–992. https://doi.org/10.1096/fj.02-0634rev
Browne S, Jha AK, Ameri K, Marcus SG, Yeghiazarians Y, Healy KE (2018) TGF-beta1/CD105 signaling controls vascular network formation within growth factor sequestering hyaluronic acid hydrogels. PLoS One 13(3):e0194679. https://doi.org/10.1371/journal.pone.0194679
Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM (1992) Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 119(3):629–641. https://doi.org/10.1083/jcb.119.3.629
Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, Schwall R, Ferrara N, Gerritsen ME (2001) Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol 158(3):1111–1120. https://doi.org/10.1016/S0002-9440(10)64058-8
Slater T, Haywood NJ, Matthews C, Cheema H, Wheatcroft SB (2019) Insulin-like growth factor binding proteins and angiogenesis: from cancer to cardiovascular disease. Cytokine Growth Factor Rev 46:28–35. https://doi.org/10.1016/j.cytogfr.2019.03.005
Haywood NJ, Slater TA, Drozd M, Warmke N, Matthews C, Cordell PA, Smith J, Rainford J, Cheema H, Maher C, Bridge KI, Yuldasheva NY, Cubbon RM, Kearney MT, Wheatcroft SB (2019) IGFBP-1 in Cardiometabolic Pathophysiology-Insights From Loss-of-Function and Gain-of-Function Studies in Male Mice. J Endocr Soc 4(1):bvz006. https://doi.org/10.1210/jendso/bvz006
Azar WJ, Azar SH, Higgins S, Hu JF, Hoffman AR, Newgreen DF, Werther GA, Russo VC (2011) IGFBP-2 enhances VEGF gene promoter activity and consequent promotion of angiogenesis by neuroblastoma cells. Endocrinology 152(9):3332–3342. https://doi.org/10.1210/en.2011-1121
Das SK, Bhutia SK, Azab B, Kegelman TP, Peachy L, Santhekadur PK, Dasgupta S, Dash R, Dent P, Grant S, Emdad L, Pellecchia M, Sarkar D, Fisher PB (2013) MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res 73(2):844–854. https://doi.org/10.1158/0008-5472.CAN-12-1681
Granata R, Trovato L, Lupia E, Sala G, Settanni F, Camussi G, Ghidoni R, Ghigo E. Insulin-like growth factor binding protein-3 induces angiogenesis through IGF-I- and SphK1-dependent mechanisms. J Thromb Haemost (2007) 5(4):835–845. https://doi.org/10.1111/j.1538-7836.2007.02431.x
Dallinga MG, Habani YI, Kayser RP, Van Noorden CJF, Klaassen I, Schlingemann RO (2020) IGF-binding proteins 3 and 4 are regulators of sprouting angiogenesis. Mol Biol Rep 47(4):2561–2572. https://doi.org/10.1007/s11033-020-05339-0
Zhuge X, Murayama T, Arai H, Yamauchi R, Tanaka M, Shimaoka T, Yonehara S, Kume N, Yokode M, Kita T (2005) CXCL16 is a novel angiogenic factor for human umbilical vein endothelial cells. Biochem Biophys Res Commun 331(4):1295–1300. https://doi.org/10.1016/j.bbrc.2005.03.200
Yu X, Zhao R, Lin S, Bai X, Zhang L, Yuan S, Sun L (2016) CXCL16 induces angiogenesis in autocrine signaling pathway involving hypoxia-inducible factor 1alpha in human umbilical vein endothelial cells. Oncol Rep 35(3):1557–1565. https://doi.org/10.3892/or.2015.4520
Ferrari G, Cook BD, Terushkin V, Pintucci G, Mignatti P (2009) Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J Cell Physiol 219(2):449–458. https://doi.org/10.1002/jcp.21706
Budi EH, Mamai O, Hoffman S, Akhurst RJ, Derynck R (2019) Enhanced TGF-beta Signaling Contributes to the Insulin-Induced Angiogenic Responses of Endothelial Cells. iScience 11:474–491. https://doi.org/10.1016/j.isci.2018.12.038
Stepanova V, Jayaraman PS, Zaitsev SV, Lebedeva T, Bdeir K, Kershaw R, Holman KR, Parfyonova YV, Semina EV, Beloglazova IB, Tkachuk VA, Cines DB (2016) Urokinase-type Plasminogen Activator (uPA) Promotes Angiogenesis by Attenuating Proline-rich Homeodomain Protein (PRH) Transcription Factor Activity and De-repressing Vascular Endothelial Growth Factor (VEGF) Receptor Expression. J Biol Chem 291(29):15029–15045. https://doi.org/10.1074/jbc.M115.678490
van Hinsbergh VW, Engelse MA, Quax PH (2006) Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol 26(4):716–728. https://doi.org/10.1161/01.ATV.0000209518.58252.17
Blackburn JS, Brinckerhoff CE (2008) Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am J Pathol 173(6):1736–1746. https://doi.org/10.2353/ajpath.2008.080512
Fan Y, Ye J, Shen F, Zhu Y, Yeghiazarians Y, Zhu W, Chen Y, Lawton MT, Young WL, Yang GY (2008) Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab 28(1):90–98. https://doi.org/10.1038/sj.jcbfm.9600509
Kayakabe K, Kuroiwa T, Sakurai N, Ikeuchi H, Kadiombo AT, Sakairi T, Matsumoto T, Maeshima A, Hiromura K, Nojima Y (2012) Interleukin-6 promotes destabilized angiogenesis by modulating angiopoietin expression in rheumatoid arthritis. Rheumatology (Oxford) 51(9):1571–1579. https://doi.org/10.1093/rheumatology/kes093
Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG (2003) Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem 278(10):8508–8515. https://doi.org/10.1074/jbc.M208231200
Li A, Dubey S, Varney ML, Dave BJ, Singh RK (2003) IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 170(6):3369–3376. https://doi.org/10.4049/jimmunol.170.6.3369
Hong KH, Ryu J, Han KH (2005) Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 105(4):1405–1407. https://doi.org/10.1182/blood-2004-08-3178
Stamatovic SM, Keep RF, Mostarica-Stojkovic M, Andjelkovic AV (2006) CCL2 regulates angiogenesis via activation of Ets-1 transcription factor. J Immunol 177(4):2651–2661. https://doi.org/10.4049/jimmunol.177.4.2651
Lobov IB, Brooks PC, Lang RA (2002) Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA 99(17):11205–11210. https://doi.org/10.1073/pnas.172161899
Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, Kutschera S, Bartels S, Appak S, Besemfelder E, Terhardt D, Chavakis E, Wieland T, Klein C, Thomas M, Uemura A, Goerdt S, Augustin HG (2012) Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 122(6):1991–2005. https://doi.org/10.1172/JCI58832
Nör JE, Mitra RS, Sutorik MM, Mooney DJ, Castle VP, Polverini PJ (2000) Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J Vasc Res 37(3):209–218. https://doi.org/10.1159/000025733
Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD (2010) Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem 285(50):38923–38932. https://doi.org/10.1074/jbc.M110.172304
Xu X, Mao W, Chen Q, Zhuang Q, Wang L, Dai J, Wang H, Huang Z (2014) Endostar, a modified recombinant human endostatin, suppresses angiogenesis through inhibition of Wnt/beta-catenin signaling pathway. PLoS One 9(9):e107463. https://doi.org/10.1371/journal.pone.0107463
Ergün S, Kilic N, Wurmbach JH, Ebrahimnejad A, Fernando M, Sevinc S, Kilic E, Chalajour F, Fiedler W, Lauke H, Lamszus K, Hammerer P, Weil J, Herbst H, Folkman J (2001) Endostatin inhibits angiogenesis by stabilization of newly formed endothelial tubes. Angiogenesis 4(3):193–206. https://doi.org/10.1023/a:1014027218980
Ikenaka Y, Yoshiji H, Kuriyama S, Yoshii J, Noguchi R, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Masaki T, Fukui H (2003) Tissue inhibitor of metalloproteinases-1 (TIMP-1) inhibits tumor growth and angiogenesis in the TIMP-1 transgenic mouse model. Int J Cancer 105(3):340–346. https://doi.org/10.1002/ijc.11094
Reed MJ, Koike T, Sadoun E, Sage EH, Puolakkainen P (2003) Inhibition of TIMP1 enhances angiogenesis in vivo and cell migration in vitro. Microvasc Res 65(1):9–17. https://doi.org/10.1016/s0026-2862(02)00026-2
Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG (2003) TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell 114(2):171–180. https://doi.org/10.1016/s0092-8674(03)00551-8
Lahat N, Bitterman H, Engelmayer-Goren M, Rosenzweig D, Weiss-Cerem L, Rahat MA (2011) Reduced TIMP-2 in hypoxia enhances angiogenesis. Am J Physiol Cell Physiol 300(3):C557–C566. https://doi.org/10.1152/ajpcell.00177.2010
Wu J, Strawn TL, Luo M, Wang L, Li R, Ren M, Xia J, Zhang Z, Ma W, Luo T, Lawrence DA, Fay WP (2015) Plasminogen activator inhibitor-1 inhibits angiogenic signaling by uncoupling vascular endothelial growth factor receptor-2-alphaVbeta3 integrin cross talk. Arterioscler Thromb Vasc Biol 35(1):111–120. https://doi.org/10.1161/ATVBAHA.114.304554
Bajou K, Herkenne S, Thijssen VL, D’Amico S, Nguyen NQ, Bouché A, Tabruyn S, Srahna M, Carabin JY, Nivelles O, Paques C, Cornelissen I, Lion M, Noel A, Gils A, Vinckier S, Declerck PJ, Griffioen AW, Dewerchin M, Martial JA, Carmeliet P, Struman I (2014) PAI-1 mediates the antiangiogenic and profibrinolytic effects of 16K prolactin. Nat Med 20(7):741–747. https://doi.org/10.1038/nm.3552
Kutikhin AG, Velikanova EA, Mukhamadiyarov RA, Glushkova TV, Borisov VV, Matveeva VG, Antonova LV, Filip’ev DE, Golovkin AS, Shishkova DK, Burago AY, Frolov AV, Dolgov VY, Efimova OS, Popova AN, Malysheva VY, Vladimirov AA, Sozinov SA, Ismagilov ZR, Russakov DM, Lomzov AA, Pyshnyi DV, Gutakovsky AK, Zhivodkov YA, Demidov EA, Peltek SE, Dolganyuk VF, Babich OO, Grigoriev EV, Brusina EB, Barbarash OL, Yuzhalin AE (2016) Apoptosis-mediated endothelial toxicity but not direct calcification or functional changes in anti-calcification proteins defines pathogenic effects of calcium phosphate bions. Sci Rep 6:27255. https://doi.org/10.1038/srep27255
Shishkova D, Velikanova E, Sinitsky M, Tsepokina A, Gruzdeva O, Bogdanov L, Kutikhin A (2019) Calcium Phosphate Bions Cause Intimal Hyperplasia in Intact Aortas of Normolipidemic Rats through Endothelial Injury. Int J Mol Sci 20(22):5728. https://doi.org/10.3390/ijms20225728
Shishkova D, Markova V, Sinitsky M, Tsepokina A, Velikanova E, Bogdanov L, Glushkova T, Kutikhin A (2020) Calciprotein Particles Cause Endothelial Dysfunction under Flow. Int J Mol Sci 21(22):8802. https://doi.org/10.3390/ijms21228802
Shishkova DK, Velikanova EA, Bogdanov LA, Sinitsky MY, Kostyunin AE, Tsepokina AV, Gruzdeva OV, Mironov AV, Mukhamadiyarov RA, Glushkova TV, Krivkina EO, Matveeva VG, Hryachkova ON, Markova VE, Dyleva YA, Belik EV, Frolov AV, Shabaev AR, Efimova OS, Popova AN, Malysheva VY, Kolmykov RP, Sevostyanov OG, Russakov DM, Dolganyuk VF, Gutakovsky AK, Zhivodkov YA, Kozhukhov AS, Brusina EB, Ismagilov ZR, Barbarash OL, Yuzhalin AE, Kutikhin AG (2021) Calciprotein Particles Link Disturbed Mineral Homeostasis with Cardiovascular Disease by Causing Endothelial Dysfunction and Vascular Inflammation. Int J Mol Sci 22(22):12458. https://doi.org/10.3390/ijms222212458
Kutikhin AG, Feenstra L, Kostyunin AE, Yuzhalin AE, Hillebrands JL, Krenning G (2021) Calciprotein Particles: Balancing Mineral Homeostasis and Vascular Pathology. Arterioscler Thromb Vasc Biol 41(5):1607–1624. https://doi.org/10.1161/ATVBAHA.120.315697
Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N (2002) VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci USA 99(14):9462–9467. https://doi.org/10.1073/pnas.142224299
Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC (2003) Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res 93(4):354–363. https://doi.org/10.1161/01.RES.0000089257.94002.96.34
Lee HJ, Koh GY (2003) Shear stress activates Tie2 receptor tyrosine kinase in human endothelial cells. Biochem Biophys Res Commun 304(2):399–404. https://doi.org/10.1016/S0006-291X(03)00592-8
Osawa M, Masuda M, Kusano K, Fujiwara K (2002) Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cellmechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol 158(4):773–785. https://doi.org/10.1083/jcb.200205049
Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA (2005) A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437(7057):426–431. https://doi.org/10.1038/nature03952
Ishida T, Peterson TE, Kovach NL, Berk BC (1996) MAP kinase activation by flow in endothelial cells. Role of beta 1 integrins and tyrosine kinases. Circ Res 79(2):310–316. https://doi.org/10.1161/01.RES.79.2.310
Li S, Kim M, Hu YL, Jalali S, Schlaepfer DD, Hunter T, Chien S, Shyy JY (1997) Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem 272(48):30455–30462. https://doi.org/10.1074/jbc.272.48.30455
Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S (2001) Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci USA 98(3):1042–1046. https://doi.org/10.1073/pnas.031562998
Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA (2001) Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J 20(17):4639–4647. https://doi.org/10.1093/emboj/20.17.4639
Pang Z, Antonetti DA, Tarbell JM (2005) Shear stress regulates HUVEC hydraulic conductivity by occludin phosphorylation. Ann Biomed Eng 33(11):1536–1545. https://doi.org/10.1007/s10439-005-7786-0
Brakemeier S, Eichler I, Hopp H, Köhler R, Hoyer J (2002) Up-regulation of endothelial stretch-activated cation channels by fluid shear stress. Cardiovasc Res 53(1):209–218. https://doi.org/10.1016/S0008-6363(01)00476-X
Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li YS, Chien S, Patapoutian A (2014) Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA 111(28):10347–10352. https://doi.org/10.1073/pnas.1409233111
Baeyens N, Mulligan-Kehoe MJ, Corti F, Simon DD, Ross TD, Rhodes JM, Wang TZ, Mejean CO, Simons M, Humphrey J, Schwartz MA (2014) Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc Natl Acad Sci USA 111(48):17308–17313. https://doi.org/10.1073/pnas.1413725111
Chachisvilis M, Zhang YL, Frangos JA (2006) G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA 103(42):15463–15468. https://doi.org/10.1073/pnas.0607224103
Gudi S, Nolan JP, Frangos JA (1998) Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci U S A 95(5):2515–2519. https://doi.org/10.1073/pnas.95.5.251
Gudi S, Huvar I, White CR, McKnight NL, Dusserre N, Boss GR, Frangos JA (2003) Rapid activation of Ras by fluid flow is mediated by Galpha(q) and Gbetagamma subunits of heterotrimeric G proteins in human endothelial cells. Arterioscler Thromb Vasc Biol 23(6):994–1000. https://doi.org/10.1161/01.ATV.0000073314.51987.84
Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM (2003) Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 93(10):e136–142. https://doi.org/10.1161/01.RES.0000101744.47866.D5
Lopez-Quintero SV, Cancel LM, Pierides A, Antonetti D, Spray DC, Tarbell JM (2013) High glucose attenuates shear-induced changes in endothelial hydraulic conductivity by degrading the glycocalyx. PLoS One 8(11):e78954. https://doi.org/10.1371/journal.pone.0078954
Isshiki M, Ando J, Korenaga R, Kogo H, Fujimoto T, Fujita T, Kamiya A (1998) Endothelial Ca2+ waves preferentially originate at specific loci in caveolin-rich cell edges. Proc Natl Acad Sci U S A 95(9):5009–5014. https://doi.org/10.1073/pnas.95.9.5009
Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC (2006) Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest 116(5):1284–1291. https://doi.org/10.1172/JCI27100
Iomini C, Tejada K, Mo W, Vaananen H, Piperno G (2004) Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol 164(6):811–817. https://doi.org/10.1083/jcb.200312133
AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM (2009) Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res 104(7):860–869. https://doi.org/10.1161/CIRCRESAHA.108.192765
Helmke BP, Goldman RD, Davies PF (2000) Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ Res 86(7):745–752. https://doi.org/10.1161/01.RES.86.7.745
Helmke BP, Thakker DB, Goldman RD, Davies PF (2001) Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophys J 80(1):184–194. https://doi.org/10.1016/S0006-3495(01)76006-7
Malek AM, Izumo S (1996) Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci 109 (Pt 4):713–726. https://doi.org/10.1242/jcs.109.4.713
Satcher R, Dewey CF Jr, Hartwig JH (1997) Mechanical remodeling of the endothelial surface and actin cytoskeleton induced by fluid flow. Microcirculation 4(4):439–453. https://doi.org/10.3109/10739689709146808
Osborn EA, Rabodzey A, Dewey CF Jr, Hartwig JH (2006) Endothelial actin cytoskeleton remodeling during mechanostimulation with fluid shear stress. Am J Physiol Cell Physiol 290(2):C444–C452. https://doi.org/10.1152/ajpcell.00218.2005
Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ (2005) Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone regulating genes. Am J Pathol 167(2):609–618. https://doi.org/10.1016/S0002-9440(10)63002-7
Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, Elderkamp YW, Meijers JC, Voorberg J, Pannekoek H, Horrevoets AJ (2006) KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood 107(11):4354–4363. https://doi.org/10.1182/blood-2005-08-3465
Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA Jr, García-Cardeña G (2006) Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest 116(1):49–58. https://doi.org/10.1172/JCI24787
Villarreal G Jr, Zhang Y, Larman HB, Gracia-Sancho J, Koo A, García-Cardeña G (2010) Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun 391(1):984–989. https://doi.org/10.1016/j.bbrc.2009.12.002
Zhou G, Hamik A, Nayak L, Tian H, Shi H, Lu Y, Sharma N, Liao X, Hale A, Boerboom L, Feaver RE, Gao H, Desai A, Schmaier A, Gerson SL, Wang Y, Atkins GB, Blackman BR, Simon DI, Jain MK (2012) Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest 122(12):4727–4731. https://doi.org/10.1172/JCI66056
Jiang YZ, Jiménez JM, Ou K, McCormick ME, Zhang LD, Davies PF (2014) Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-Like Factor 4 promoter in vitro and in vivo. Circ Res 115(1):32–43. https://doi.org/10.1161/CIRCRESAHA.115.303883
Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA Jr (2007) Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res 101(7):723–733. https://doi.org/10.1161/CIRCRESAHA.107.152942
Zakkar M, Van der Heiden K, Luong le A, Chaudhury H, Cuhlmann S, Hamdulay SS, Krams R, Edirisinghe I, Rahman I, Carlsen H, Haskard DO, Mason JC, Evans PC (2009) Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol 29(11):1851–1857. https://doi.org/10.1161/ATVBAHA.109.193375
Wang KC, Yeh YT, Nguyen P, Limqueco E, Lopez J, Thorossian S, Guan KL, Li YJ, Chien S (2016) Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci U S A 113(41):11525–11530. https://doi.org/10.1073/pnas.1613121113
Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, Liu J, Deng D, Lau CW, Wan S, Ai D, Mak KK, Tong KK, Kwan KM, Wang N, Chiu JJ, Zhu Y, Huang Y (2016) Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 540(7634):579–582. https://doi.org/10.1038/nature20602
SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA Jr, García-Cardeña G, Jain MK (2004) KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med 199(10):1305–1315. https://doi.org/10.1084/jem.20031132
Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA Jr, Balasubramanian V, García-Cardeña G, Jain MK (2005) Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res 96(5):e48–e57. https://doi.org/10.1161/01.RES.0000159707.05637.a1
van Thienen JV, Fledderus JO, Dekker RJ, Rohlena J, van Ijzendoorn GA, Kootstra NA, Pannekoek H, Horrevoets AJ (2006) Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc Res 72(2):231–240. https://doi.org/10.1016/j.cardiores.2006.07.008
Davies PF, Civelek M, Fang Y, Fleming I (2013) The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 99(2):315–327. https://doi.org/10.1093/cvr/cvt101
Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C (2003) Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem 278(2):703–711. https://doi.org/10.1074/jbc.M203161200
Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Warabi E, Noguchi N, Itoh K, Yamamoto M (2005) Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem 280(29):27244–27250. https://doi.org/10.1074/jbc.M502551200
Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, Kunsch C (2006) Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol 290(5):H1862–H1870. https://doi.org/10.1152/ajpheart.00651.2005
Fledderus JO, Boon RA, Volger OL, Hurttila H, Ylä-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJ (2008) KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol 28(7):1339–1346. https://doi.org/10.1161/ATVBAHA.108.165811
Lazaro I, Lopez-Sanz L, Bernal S, Oguiza A, Recio C, Melgar A, Jimenez-Castilla L, Egido J, Madrigal-Matute J, Gomez-Guerrero C (2018) Nrf2 Activation Provides Atheroprotection in Diabetic Mice Through Concerted Upregulation of Antioxidant, Anti-inflammatory, and Autophagy Mechanisms. Front Pharmacol 9:819. https://doi.org/10.3389/fphar.2018.00819
Nakajima H, Mochizuki N. Flow pattern-dependent endothelial cell responses through transcriptional regulation (2017) Cell Cycle 16(20):1893–1901. https://doi.org/10.1080/15384101.2017.1364324
Panciera T, Azzolin L, Cordenonsi M, Piccolo S (2017) Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol 18(12):758–770. https://doi.org/10.1038/nrm.2017.87
Yu Y, Su X, Qin Q, Hou Y, Zhang X, Zhang H, Jia M, Chen Y (2020) Yes-associated protein and transcriptional coactivator with PDZ-binding motif as new targets in cardiovascular diseases. Pharmacol Res 159:105009. https://doi.org/10.1016/j.phrs.2020.105009
Yuan P, Hu Q, He X, Long Y, Song X, Wu F, He Y, Zhou X (2020) Laminar flow inhibits the Hippo/YAP pathway via autophagy and SIRT1-mediated deacetylation against atherosclerosis. Cell Death Dis 11(2):141. https://doi.org/10.1038/s41419-020-2343-1
Moonen JR, Lee ES, Schmidt M, Maleszewska M, Koerts JA, Brouwer LA, van Kooten TG, van Luyn MJ, Zeebregts CJ, Krenning G, Harmsen MC (2015) Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc Res 108(3):377–386. https://doi.org/10.1093/cvr/cvv175
Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M (2015) Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest 125(12):4514–4528. https://doi.org/10.1172/JCI82719
Mahmoud MM, Serbanovic-Canic J, Feng S, Souilhol C, Xing R, Hsiao S, Mammoto A, Chen J, Ariaans M, Francis SE, Van der Heiden K, Ridger V, Evans PC (2017) Shear stress induces endothelial-to-mesenchymal transition via the transcription factor Snail. Sci Rep 7(1):3375. https://doi.org/10.1038/s41598-017-03532-z
Evrard SM, Lecce L, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman KR, d’Escamard V, Li JR, Hadri L, Fujitani K, Moreno PR, Benard L, Rimmele P, Cohain A, Mecham B, Randolph GJ, Nabel EG, Hajjar R, Fuster V, Boehm M, Kovacic JC (2016) Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun 7:11853. https://doi.org/10.1038/ncomms11853
Souilhol C, Harmsen MC, Evans PC, Krenning G (2018) Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc Res 114(4):565–577. https://doi.org/10.1093/cvr/cvx253
Dejana E, Hirschi KK, Simons M (2017) The molecular basis of endothelial cell plasticity. Nat Commun 8:14361. https://doi.org/10.1038/ncomms14361
Jackson AO, Zhang J, Jiang Z, Yin K (2017) Endothelial-to-mesenchymal transition: A novel therapeutic target for cardiovascular diseases. Trends Cardiovasc Med 27(6):383–393. https://doi.org/10.1016/j.tcm.2017.03.003
Cho JG, Lee A, Chang W, Lee MS, Kim J (2018) Endothelial to Mesenchymal Transition Represents a Key Link in the Interaction between Inflammation and Endothelial Dysfunction. Front Immunol 9:294. https://doi.org/10.3389/fimmu.2018.0029
Schwartz MA, Vestweber D, Simons M (2018) A unifying concept in vascular health and disease. Science 360(6386):270–271. https://doi.org/10.1126/science.aat3470
Chen PY, Schwartz MA, Simons M (2020) Endothelial-to-Mesenchymal Transition, Vascular Inflammation, and Atherosclerosis. Front Cardiovasc Med 7:53. https://doi.org/10.3389/fcvm.2020.00053
Sánchez-Duffhues G, García de Vinuesa A, van de Pol V, Geerts ME, de Vries MR, Janson SG, van Dam H, Lindeman JH, Goumans MJ, Ten Dijke P (2019) Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J Pathol 247(3):333–346. https://doi.org/10.1002/path.5193
Mehta V, Pang KL, Givens CS, Chen Z, Huang J, Sweet DT, Jo H, Reader JS, Tzima E (2021) Mechanical forces regulate endothelial-to-mesenchymal transition and atherosclerosis via an Alk5-Shc mechanotransduction pathway. Sci Adv 7(28):eabg5060. https://doi.org/10.1126/sciadv.abg5060
Egorova AD, Khedoe PP, Goumans MJ, Yoder BK, Nauli SM, ten Dijke P, Poelmann RE, Hierck BP (2011) Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res 108(9):1093–1101. https://doi.org/10.1161/CIRCRESAHA.110.231860
Libby P, Lüscher T (2020) COVID-19 is, in the end, an endothelial disease. Eur Heart J 41(32):3038–3044. https://doi.org/10.1093/eurheartj/ehaa623
Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, Englert H, Byrne M, Bergin C, O'Sullivan JM, Martin-Loeches I, Nadarajan P, Bannan C, Mallon PW, Curley GF, Preston RJS, Rehill AM, McGonagle D, Ni Cheallaigh C, Baker RI, Renné T, Ward SE, O'Donnell JS, Irish COVID-19 Vasculopathy Study (iCVS) investigators (2021) Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost 19(10):2546–2553. https://doi.org/10.1111/jth.15490
Gorog DA, Storey RF, Gurbel PA, Tantry US, Berger JS, Chan MY, Duerschmied D, Smyth SS, Parker WAE, Ajjan RA, Vilahur G, Badimon L, Berg JMT, Cate HT, Peyvandi F, Wang TT, Becker RC (2022) Current and novel biomarkers of thrombotic risk in COVID-19: a Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat Rev Cardiol 19:475–495. https://doi.org/10.1038/s41569-021-00665-7
Funding
This study was funded by the Ministry of Science and Higher Education of the Russian Federation (National Project Science and Universities, Research Topic No. 0419-2021-001).
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design—A.G.K., critical analysis—A.G.K., D.K.Sh., E.A.V., M.Yu.S., A.V.S., literature data analysis— E.A.V., M.Yu.S., A.V.S., V.E.M., article writing—A.G.K., D.K.Sh.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have neither evident nor potential conflict of interest as related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 5, pp. 594–625https://doi.org/10.31857/S0869813922050077.
Rights and permissions
About this article
Cite this article
Kutikhin, A.G., Shishkova, D.K., Velikanova, E.A. et al. Endothelial Dysfunction in the Context of Blood–Brain Barrier Modeling. J Evol Biochem Phys 58, 781–806 (2022). https://doi.org/10.1134/S0022093022030139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022030139