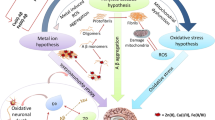
Abstract
Methylene blue (MB) is the first fully synthetic compound that had found its way into medicine over 120 years ago as a treatment against malaria. MB has been approved for the treatment of methemoglobinemia, but there are premises for its repurposing as a neuroprotective agent based on the efficacy of this compound demonstrated in the models of Alzheimer’s, Parkinson’s, and Huntington’s diseases, traumatic brain injury, amyotrophic lateral sclerosis, depressive disorders, etc. However, the goal of this review was not so much to focus on the therapeutic effects of MB in the treatment of various neurodegeneration diseases, but to delve into the mechanisms of direct or indirect effect of this drug on the signaling pathways. MB can act as an alternative electron carrier in the mitochondrial respiratory chain in the case of dysfunctional electron transport chain. It also displays the anti-inflammatory and anti-apoptotic effects, inhibits monoamine oxidase (MAO) and nitric oxide synthase (NOS), activates signaling pathways involved in the mitochondrial pool renewal (mitochondrial biogenesis and autophagy), and prevents aggregation of misfolded proteins. Comprehensive understanding of all aspects of direct and indirect influence of MB, and not just some of its effects, can help in further research of this compound, including its clinical applications.







Similar content being viewed by others
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- ARE:
-
antioxidant response element
- cGMP:
-
cyclic guanosine monophosphate
- CRH:
-
corticotropin-releasing hormone
- ETC:
-
electron transport chain
- GSK3β:
-
glycogen synthase kinase-3 beta
- HTT:
-
huntingtin
- Keap1:
-
Kelch-like ECH-associated protein 1
- MAO:
-
monoamine oxidase
- MB:
-
methylene blue
- mTORC1:
-
mammalian target of rapamycin complex 1
- NF-κB:
-
nuclear factor kappa-B
- NOS:
-
nitric oxide synthase
- Nrf2:
-
nuclear factor erythroid 2-related factor 2
- PGC-1α:
-
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PI3K:
-
phosphoinositide 3-kinase
- PMCA:
-
plasma membrane Ca2+-ATPase
- ROS:
-
reactive oxygen species
- sGC:
-
soluble guanylate cyclase
- SOD1:
-
superoxide dismutase 1
- STAT:
-
signal transducer and transcription activator
- TNF-α:
-
tumor necrosis factor
References
O’Neil, M. J. (2013) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, Cambridge, UK, Royal Society of Chemistry, England.
Deutsches Reich Patent no. 1886, December 15, 1877 Badische Anilin- und Sodafabrik [BASF] (Mannheim, Germany), “Verfahren zur Darstellung blauer Farbstoffe aus Dimethylanilin und anderen tertiaren aromatischen Monaminen” (Method for preparation of blue dyes from dimethylaniline and other tertiary aromatic monoamines), Deutsches Reich Patent no. 1886 (December 15, 1877).
Berneth, H. (2008) Azine dyes. In: Ullmann’s Encyclopedia of Industrial Chemistry, Weinheim, Germany.
Delport, A., Harvey, B. H., Petzer, A., and Petzer, J. P. (2017) The monoamine oxidase inhibition properties of selected structural analogues of methylene blue, Toxicol. Appl. Pharmacol., 325, 1-8, https://doi.org/10.1016/j.taap.2017.03.026.
Gaudette, N. F., and Lodge, J. W. (2005) Determination of methylene blue and leucomethylene blue in male and female Fischer 344 rat urine and B6C3F1 mouse urine, J. Anal. Toxicol., 29, 28-33, https://doi.org/10.1093/jat/29.1.28.
Schirmer, R. H., Adler, H., Pickhardt, M., and Mandelkow, E. (2011) “Lest we forget you – methylene blue...”, Neurobiol. Aging, 32, 2325.e7-2325.e2.325E16, https://doi.org/10.1016/j.neurobiolaging.2010.12.012.
Ehrlich, P., and Leppmann, A., (1890) Ueber schmerzstillende Wirkung des Methylenblau, Dtsch. Med. Wochenschr., 16, 493-494.
Bodoni, P. (1899) Dell’azione sedativa del bleu di metilene in varie forme di psicosi [in Italian], Clin. Med. Ital., pp. 217-222.
Ohlow, M. J., and Moosmann, B. (2011) Phenothiazine: the seven lives of pharmacology's first lead structure, Drug Discov. Today, 16, 119-131, https://doi.org/10.1016/j.drudis.2011.01.001.
Allexsaht, W. J. (1938) The use of methylene blue in the treatment of catatonic dementia praecox patients, Psychiatric Quarterly, 12, 245-252.
Kajdi, L., and Taylor, C. V. (1938) The use of intravenous methylene blue in status convulsivus, Am. J. Insanity, 94, 1369-1376, https://doi.org/10.1176/ajp.94.6.1369.
Naylor, G. J., and Smith, A. H. (1981) Vanadium: a possible aetiological factor in manic depressive illness, Psychol. Med., 11, 249-256, https://doi.org/10.1017/s0033291700052065.
Naylor, G. J., Martin, B., Hopwood, S. E., and Watson, Y. (1986) A two-year double-blind crossover trial of the prophylactic effect of methylene blue in manic depressive psychosis, Biol. Psychiatry, 21, 915-920, https://doi.org/10.1016/0006-3223(86)90265-9.
Martinez, J. L., Jensen, R. A., Vasquez, B. J., McGuinness, T., and McGaugh, J. L. (1978) Methylene blue alters retention of inhibitory avoidance responses, Psychobiology, 6, 387-390, https://doi.org/10.3758/BF03326744.
Wischik, C. M., Edwards, P. C., Lai, R. Y., Roth, M., and Harrington, C. R. (1996) Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines, Proc. Natl. Acad. Sci. USA, 93, 11213-11218, https://doi.org/10.1073/pnas.93.20.11213.
Wischik, C. M., Staff, R. T., Wischik, D. J., Bentham, P., Murray, A. D., et al. (2015) Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease, J. Alzheimer’s Dis., 44, 705-720, https://doi.org/10.3233/JAD-142874.
Gauthier, S., Feldman, H. H., Schneider, L. S., Wilcock, G. K., Frisoni, G. B., et al. (2016) Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer's disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial, Lancet, 388, 2873-2884, https://doi.org/10.1016/S0140-6736(16)31275-2.
Hashweh, N. N., Bartochowski, Z., Khoury, R., and Grossberg, G. T. (2020) An evaluation of hydromethylthionine as a treatment option for Alzheimer’s disease, Expert Opin. Pharmacother., 21, 619-627, https://doi.org/10.1080/14656566.2020.1719066.
Sontag, E. M., Lotz, G. P., Agrawal, N., Tran, A., Aron, R., et al. (2012) Methylene blue modulates huntingtin aggregation intermediates and is protective in Huntington’s disease models, J. Neurosci., 32, 11109-11119, https://doi.org/10.1523/JNEUROSCI.0895-12.2012.
Heidari, R., Monnier, V., Martin, E., and Tricoire, H. (2015) Methylene blue partially rescues heart defects in a drosophila model of Huntington’s disease, J. Huntington’s Dis., 4, 173-186, https://doi.org/10.3233/JHD-140130.
Bariotto-Dos-Santos, K., Padovan-Neto, F. E., Bortolanza, M., Dos-Santos-Pereira, M., Raisman-Vozari, R., et al. (2019) Repurposing an established drug: an emerging role for methylene blue in L-DOPA-induced dyskinesia, Eur. J. Neurosci., 49, 869-882, https://doi.org/10.1111/ejn.14079.
Bhurtel, S., Katila, N., Neupane, S., Srivastav, S., Park, P. H., et al. (2018) Methylene blue protects dopaminergic neurons against MPTP-induced neurotoxicity by upregulating brain-derived neurotrophic factor, Ann. NY Acad. Sci., 1431, 58-71, https://doi.org/10.1111/nyas.13870.
Stelmashook, E. V., Genrikhs, E. E., Mukhaleva, E. V., Kapkaeva, M. R., Kondratenko, R. V., et al. (2019) Neuroprotective effects of methylene blue in vivo and in vitro, Bull. Exp. Biol. Med., 167, 455-459, https://doi.org/10.1007/s10517-019-04548-3.
Genrikhs, E. E., Stelmashook, E. V., Voronkov, D. N., Novikova, S. V., Alexandrova, O. P., et al. (2020) The delayed neuroprotective effect of methylene blue in experimental rat brain trauma, Antioxidants, 9, 377, https://doi.org/10.3390/antiox9050377.
Genrikhs, E. E., Stelmashook, E. V., Voronkov, D. N., Novikova, S. V., Alexandrova, O. P., et al. (2020) The single intravenous administration of methylene blue after traumatic brain injury diminishes neurological deficit, blood-brain barrier disruption and decrease in the expression of S100 protein in rats, Brain Res., 1740, 146854, https://doi.org/10.1016/j.brainres.2020.146854.
Lu, Q., Tucker, D., Dong, Y., Zhao, N., and Zhang, Q. (2016) Neuroprotective and functional improvement effects of methylene blue in global cerebral ischemia, Mol. Neurobiol., 53, 5344-5355, https://doi.org/10.1007/s12035-015-9455-0.
Li, L., Yang, R., Li, P., Lu, H., Hao, J., et al. (2018) Combination treatment with methylene blue and hypothermia in global cerebral ischemia, Mol. Neurobiol., 55, 2042-2055, https://doi.org/10.1007/s12035-017-0470-1.
Lin, Z. H., Wang, S. Y., Chen, L. L., Zhuang, J. Y., Ke, Q. F., et al. (2017) Methylene blue mitigates acute neuroinflammation after spinal cord injury through inhibiting NLRP3 inflammasome activation in microglia, Front. Cell Neurosci., 11, 391, https://doi.org/10.3389/fncel.2017.00391.
Dibaj, P., Zschüntzsch, J., Steffens, H., Scheffel, J., Göricke, B., et al. (2012) Influence of methylene blue on microglia-induced inflammation and motor neuron degeneration in the SOD1(G93A) model for ALS, PLoS One, 7, e43963, https://doi.org/10.1371/journal.pone.0043963.
Zeevalk, G. D., Bernard, L. P., Song, C., Gluck, M., and Ehrhart, J. (2005) Mitochondrial inhibition and oxidative stress: reciprocating players in neurodegeneration, Antioxid. Redox Signal., 7, 1117-1139, https://doi.org/10.1089/ars.2005.7.1117.
Andreyev, A. Y., Kushnareva, Y. E., and Starkov, A. A. (2005) Mitochondrial metabolism of reactive oxygen species, Biochemistry (Moscow), 70, 200-214, https://doi.org/10.1007/s10541-005-0102-7.
Eubel, H., Heinemeyer, J., Sunderhaus, S., and Braun, H. P. (2004) Respiratory chain supercomplexes in plant mitochondria, Plant Physiol. Biochem., 42, 937-942, https://doi.org/10.1016/j.plaphy.2004.09.010.
Cadenas, S. (2018) Mitochondrial uncoupling, ROS generation and cardioprotection, Biochim. Biophys. Acta Bioenerg., 1859, 940-950, https://doi.org/10.1016/j.bbabio.2018.05.019.
Grundlingh, J., Dargan, P. I., El-Zanfaly, M., and Wood, D. M. (2011) 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death, J. Med. Toxicol., 7, 205-212, https://doi.org/10.1007/s13181-011-0162-6.
Wen, Y., Li, W., Poteet, E. C., Xie, L., Tan, C., et al. (2011) Alternative mitochondrial electron transfer as a novel strategy for neuroprotection, J. Biol. Chem., 286, 16504-16515, https://doi.org/10.1074/jbc.M110.208447.
Harrop, G. A., and Barron, E. S. (1928) Studies on blood cell metabolism: I. The effect of methylene blue and other dyes upon the oxygen consumption of mammalian and avian erythrocytes, J. Exp. Med., 48, 207-223, https://doi.org/10.1084/jem.48.2.207.
Barron, E. S., and Hoffman, L. A. (1930) The catalytic effect of dyes on the oxygen consumption of living cells, J. Gen. Physiol., 13, 483-494, https://doi.org/10.1085/jgp.13.4.483.
Elliott, K. A., and Greig, M. E. (1938) The distribution of the succinic oxidase system in animal tissues, Biochem. J., 32, 1407-1423, https://doi.org/10.1042/bj0321407.
Slater, E. C. (1949) A respiratory catalyst required for the reduction of cytochrome c by cytochrome b, Biochem. J., 45, 14-30, https://doi.org/10.1042/bj0450014.
Redfearn, E. R. (1961) The role of ubiquinone (coenzyme Q) and its homologues in mediating the reduction of methylene blue by succinate in heart-muscle preparations, Biochim. Biophys. Acta, 53, 581-583, https://doi.org/10.1016/0006-3002(61)90220-7.
Tönz, O. (1968) The congenital methemoglobinemias. Physiology and pathophysiology of the hemiglobin metabolism, Bibliotheca Haematologica, 28, 1-146.
Visarius, T. M., Stucki, J. W., and Lauterburg, B. H. (1997) Stimulation of respiration by methylene blue in rat liver mitochondria, FEBS Lett., 412, 157-160, https://doi.org/10.1016/s0014-5793(97)00767-9.
Gureev, A. P., Samoylova, N. A., Potanina, D. V., and Popov, V. N. (2021) Effect of methylene blue and its metabolite – azure I – on bioenergetic parameters of intact mice brain mitochondria, Biomed. Khim., 67, 485-490, https://doi.org/10.18097/PBMC20216706485.
Callaway, N. L., Riha, P. D., Wrubel, K. M., McCollum, D., and Gonzalez-Lima, F. (2002) Methylene blue restores spatial memory retention impaired by an inhibitor of cytochrome oxidase in rats, Neurosci. Lett., 332, 83-86, https://doi.org/10.1016/s0304-3940(02)00827-3.
Tretter, L., Horvath, G., Hölgyesi, A., Essek, F., and Adam-Vizi, V. (2014) Enhanced hydrogen peroxide generation accompanies the beneficial bioenergetic effects of methylene blue in isolated brain mitochondria, Free Radic. Biol. Med., 77, 317-330, https://doi.org/10.1016/j.freeradbiomed.2014.09.024.
Gureev, A. P., Syromyatnikov, M. Y., Gorbacheva, T. M., Starkov, A. A., and Popov, V. N. (2016) Methylene blue improves sensorimotor phenotype and decreases anxiety in parallel with activating brain mitochondria biogenesis in mid-age mice, Neurosci. Res., 113, 19-27, https://doi.org/10.1016/j.neures.2016.07.006.
Gureev, A. P., Shaforostova, E. A., Popov, V. N., and Starkov, A. A. (2019) Methylene blue does not bypass Complex III antimycin block in mouse brain mitochondria, FEBS Lett., 593, 499-503, https://doi.org/10.1002/1873-3468.13332.
Gureev, A. P., Shaforostova, E. A., Laver, D. A., Khorolskaya, V. G., Syromyatnikov, M. Y., et al. (2019) Methylene blue elicits non-genotoxic H2O2 production and protects brain mitochondria from rotenone toxicity, J. Appl. Biomed., 17, 107-114, https://doi.org/10.32725/jab.2019.008.
Atamna, H., Atamna, W., Al-Eyd, G., Shanower, G., and Dhahbi, J. M. (2015) Combined activation of the energy and cellular-defense pathways may explain the potent anti-senescence activity of methylene blue, Redox Biol., 6, 426-435, https://doi.org/10.1016/j.redox.2015.09.004.
Zhang, X., Rojas, J. C., and Gonzalez-Lima, F. (2006) Methylene blue prevents neurodegeneration caused by rotenone in the retina, Neurotox. Res., 9, 47-57, https://doi.org/10.1007/BF03033307.
Poteet, E., Winters, A., Yan, L. J., Shufelt, K., Green, K. N., et al. (2012) Neuroprotective actions of methylene blue and its derivatives, PLoS One, 7, e48279, https://doi.org/10.1371/journal.pone.0048279.
Mikulás, K., Komlódi, T., Földes, A., Sváb, G., Horváth, G., et al. (2020) Bioenergetic Impairment of triethylene glycol dimethacrylate- (TEGDMA-) treated dental pulp stem cells (DPSCs) and isolated brain mitochondria are amended by redox compound methylene blue, Materials, 13, 3472, https://doi.org/10.3390/ma13163472.
Sváb, G., Kokas, M., Sipos, I., Ambrus, A., and Tretter, L. (2021) Methylene blue bridges the inhibition and produces unusual respiratory changes in complex III-inhibited mitochondria. Studies on rats, mice and guinea pigs, Antioxidants, 10, 305, https://doi.org/10.3390/antiox10020305.
Irwin, M. H., Parameshwaran, K., and Pinkert, C. A. (2013) Mouse models of mitochondrial complex I dysfunction, Int. J. Biochem. Cell Biol., 45, 34-40, https://doi.org/10.1016/j.biocel.2012.08.009.
Bahn, G., and Jo, D. G. (2019) Therapeutic approaches to Alzheimer’s disease through modulation of NRF2, Neuromol. Med., 21, 1-11, https://doi.org/10.1007/s12017-018-08523-5.
Taniguchi, S., Suzuki, N., Masuda, M., Hisanaga, S., Iwatsubo, T., et al. (2005) Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins, J. Biol. Chem., 280, 7614-7623, https://doi.org/10.1074/jbc.M408714200.
Hattori, M., Sugino, E., Minoura, K., In, Y., Sumida, M., et al. (2008) Different inhibitory response of cyanidin and methylene blue for filament formation of tau microtubule-binding domain, Biochem. Biophys. Res. Commun., 374, 158-163, https://doi.org/10.1016/j.bbrc.2008.07.001.
Stack, C., Jainuddin, S., Elipenahli, C., Gerges, M., Starkova, N., et al. (2014) Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity, Hum. Mol. Genet., 23, 3716-3732, https://doi.org/10.1093/hmg/ddu080.
Hochgräfe, K., Sydow, A., Matenia, D., Cadinu, D., Könen, S., et al. (2015) Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human Tau, Acta Neuropathol. Commun., 3, 25, https://doi.org/10.1186/s40478-015-0204-4.
Necula, M., Breydo, L., Milton, S., Kayed, R., van der Veer, W. E., et al. (2007) Methylene blue inhibits amyloid Abeta oligomerization by promoting fibrillization, Biochemistry, 46, 8850-8860, https://doi.org/10.1021/bi700411k.
Lee, B. I., Suh, Y. S., Chung, Y. J., Yu, K., and Park, C. B. (2017) Shedding light on Alzheimer’s β-amyloidosis: photosensitized methylene blue inhibits self-assembly of β-amyloid peptides and disintegrates their aggregates, Sci. Rep., 7, 7523, https://doi.org/10.1038/s41598-017-07581-2.
Berrocal, M., Corbacho, I., Gutierrez-Merino, C., and Mata, A. M. (2018) Methylene blue activates the PMCA activity and cross-interacts with amyloid β-peptide, blocking Aβ-mediated PMCA inhibition, Neuropharmacology, 139, 163-172, https://doi.org/10.1016/j.neuropharm.2018.07.012.
Berrocal, M., Caballero-Bermejo, M., Gutierrez-Merino, C., and Mata, A. M. (2019) Methylene blue blocks and reverses the inhibitory effect of Tau on PMCA function, Int. J. Mol. Sci., 20, 3521, https://doi.org/10.3390/ijms20143521.
Illarioshkin, S. N., Klyushnikov, S. A., Vigont, V. A., Seliverstov, Y. A., and Kaznacheyeva, E. V. (2018) Molecular pathogenesis in Huntington’s disease, Biochemistry (Moscow), 83, 1030-1039, https://doi.org/10.1134/S0006297918090043.
Cavaliere, P., Torrent, J., Prigent, S., Granata, V., Pauwels, K., et al. (2013) Binding of methylene blue to a surface cleft inhibits the oligomerization and fibrillization of prion protein, Biochim. Biophys. Acta, 1832, 20-28, https://doi.org/10.1016/j.bbadis.2012.09.005.
Paré, B., Lehmann, M., Beaudin, M., Nordström, U., Saikali, S., et al. (2018) Misfolded SOD1 pathology in sporadic amyotrophic lateral sclerosis, Sci. Rep., 8, 14223, https://doi.org/10.1038/s41598-018-31773-z.
Yamashita, M., Nonaka, T., Arai, T., Kametani, F., Buchman, V. L., et al. (2009) Methylene blue and dimebon inhibit aggregation of TDP-43 in cellular models, FEBS Lett., 583, 2419-2424, https://doi.org/10.1016/j.febslet.2009.06.042.
Musteikyte, G., Ziaunys, M., and Smirnovas, V. (2020) Methylene blue inhibits nucleation and elongation of SOD1 amyloid fibrils, PeerJ, 8, e9719, https://doi.org/10.7717/peerj.9719.
Vaccaro, A., Patten, S. A., Ciura, S., Maios, C., Therrien, M., et al. (2012) Methylene blue protects against TDP-43 and FUS neuronal toxicity in C. elegans and D. rerio, PLoS One, 7, e42117, https://doi.org/10.1371/journal.pone.0042117.
Vaccaro, A., Patten, S. A., Aggad, D., Julien, C., Maios, C., et al. (2013) Pharmacological reduction of ER stress protects against TDP-43 neuronal toxicity in vivo, Neurobiol. Dis., 55, 64-75, https://doi.org/10.1016/j.nbd.2013.03.015.
Dinkova-Kostova, A. T., and Abramov, A. Y. (2015) The emerging role of Nrf2 in mitochondrial function, Free Radic. Biol. Med., 88, 179-188, https://doi.org/10.1016/j.freeradbiomed.2015.04.036.
Hayes, J. D., Chowdhry, S., Dinkova-Kostova, A. T., and Sutherland, C. (2015) Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of β-TrCP and GSK-3, Biochem. Soc. Trans., 43, 611-620, https://doi.org/10.1042/BST20150011.
Zenkov, N. K., Kozhin, P. M., Chechushkov, A. V., Martinovich, G. G., Kandalintseva, N. V., et al. (2017) Mazes of Nrf2 regulation, Biochemistry (Moscow), 82, 556-564, https://doi.org/10.1134/S0006297917050030.
El Sayed, N. S., and Sayed, A. S. (2019) Protective effect of methylene blue on TNBS-induced colitis in rats mediated through the modulation of inflammatory and apoptotic signalling pathways, Arch. Toxicol., 93, 2927-2942, https://doi.org/10.1007/s00204-019-02548-w.
Kaur, S., and Benov, L. T. (2020) Methylene blue induces the soxRS regulon of Escherichia coli, Chem. Biol. Interact., 329, 109222, https://doi.org/10.1016/j.cbi.2020.109222.
Gureev, A. P., Shaforostova, E. A., and Popov, V. N. (2019) Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways, Front. Genet., 10, 435, https://doi.org/10.3389/fgene.2019.00435.
Fernandez-Marcos, P. J., and Auwerx, J. (2011) Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis, Am. J. Clin. Nutr., 93, 884S-890S, https://doi.org/10.3945/ajcn.110.001917.
Islam, H., Hood, D. A., and Gurd, B. J. (2020) Looking beyond PGC-1α: emerging regulators of exercise-induced skeletal muscle mitochondrial biogenesis and their activation by dietary compounds, Appl. Physiol. Nutr. Metab., 45, 11-23, https://doi.org/10.1139/apnm-2019-0069.
Xie, L., Li, W., Winters, A., Yuan, F., Jin, K., et al. (2013) Methylene blue induces macroautophagy through 5′ adenosine monophosphate-activated protein kinase pathway to protect neurons from serum deprivation, Front. Cell Neurosci., 7, 56, https://doi.org/10.3389/fncel.2013.00056.
Kroemer, G., Mariño, G., and Levine, B. (2010) Autophagy and the integrated stress response, Mol. Cell, 40, 280-293, https://doi.org/10.1016/j.molcel.2010.09.023.
Telli, M. L., Nagata, H., Wapnir, I., Acharya, C. R., Zablotsky, K., et al. (2021) Intratumoral plasmid IL12 expands CD8+ T cells and induces a CXCR3 gene signature in triple-negative breast tumors that sensitizes patients to anti-PD-1 therapy, Clin. Cancer Res., 27, 2481-2493, https://doi.org/10.1158/1078-0432.CCR-20-3944.
Wong, E., and Cuervo, A. M. (2010) Autophagy gone awry in neurodegenerative diseases, Nat. Neurosci., 13, 805-811, https://doi.org/10.1038/nn.2575.
Medina, D. X., Caccamo, A., and Oddo, S. (2011) Methylene blue reduces aβ levels and rescues early cognitive deficit by increasing proteasome activity, Brain Pathol., 21, 140-149, https://doi.org/10.1111/j.1750-3639.2010.00430.x.
Congdon, E. E., Wu, J. W., Myeku, N., Figueroa, Y. H., Herman, M., et al. (2012) Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo, Autophagy, 8, 609-622, https://doi.org/10.4161/auto.19048.
Jiang, Z., Watts, L. T., Huang, S., Shen, Q., Rodriguez, P., et al. (2015) The effects of methylene blue on autophagy and apoptosis in MRI-defined normal tissue, ischemic penumbra and ischemic core, PLoS One, 10, e0131929, https://doi.org/10.1371/journal.pone.0131929.
Zhao, M., Liang, F., Xu, H., Yan, W., and Zhang, J. (2016) Methylene blue exerts a neuroprotective effect against traumatic brain injury by promoting autophagy and inhibiting microglial activation, Mol. Med. Rep., 13, 13-20, https://doi.org/10.3892/mmr.2015.4551.
Di, Y., He, Y. L., Zhao, T., Huang, X., Wu, K. W., et al. (2015) Methylene blue reduces acute cerebral ischemic injury via the induction of mitophagy, Mol. Med., 21, 420-429, https://doi.org/10.2119/molmed.2015.00038.
Gureev, A. P., Sadovnikova, I. S., Starkov, N. N., Starkov, A. A., and Popov, V. N. (2020) p62-Nrf2-p62 mitophagy regulatory loop as a target for preventive therapy of neurodegenerative diseases, Brain Sci., 10, 847, https://doi.org/10.3390/brainsci10110847.
Murata, H., Takamatsu, H., Liu, S., Kataoka, K., Huh, N. H., et al. (2015) NRF2 regulates PINK1 expression under oxidative stress conditions, PLoS One, 10, e0142438, https://doi.org/10.1371/journal.pone.0142438.
Dos Santos, A. F., Terra, L. F., Wailemann, R. A., Oliveira, T. C., Gomes, V. M., et al. (2017) Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells, BMC Cancer, 17, 194, https://doi.org/10.1186/s12885-017-3179-7.
Lucky, S. S., Soo, K. C., and Zhang, Y. (2015) Nanoparticles in photodynamic therapy, Chem. Rev., 115, 1990-2042, https://doi.org/10.1021/cr5004198.
Tardivo, J. P., Del Giglio, A., de Oliveira, C. S., Gabrielli, D. S., Junqueira, H. C., et al. (2005) Methylene blue in photodynamic therapy: from basic mechanisms to clinical applications, Photodiagn. Photodyn. Ther., 2, 175-191, https://doi.org/10.1016/S1572-1000(05)00097-9.
Eimon, P. M., Kratz, E., Varfolomeev, E., Hymowitz, S. G., Stern, H., et al. (2006) Delineation of the cell-extrinsic apoptosis pathway in the zebrafish, Cell Death Differ., 13, 1619-1630, https://doi.org/10.1038/sj.cdd.4402015.
Bellail, A. C., Tse, M. C., Song, J. H., Phuphanich, S., Olson, J. J., et al. (2010) DR5-mediated DISC controls caspase-8 cleavage and initiation of apoptosis in human glioblastomas, J. Cell Mol. Med., 14, 1303-1317, https://doi.org/10.1111/j.1582-4934.2009.00777.x.
Garrido, C., Galluzzi, L., Brunet, M., Puig, P. E., Didelot, C., et al. (2006) Mechanisms of cytochrome c release from mitochondria, Cell Death Differ., 13, 1423-1433, https://doi.org/10.1038/sj.cdd.4401950.
Wu, C. C., and Bratton, S. B. (2013) Regulation of the intrinsic apoptosis pathway by reactive oxygen species, Antioxid Redox Signal., 19, 546-558, https://doi.org/10.1089/ars.2012.4905.
Roy, S., and Nicholson, D. W. (2000) Cross-talk in cell death signaling, J. Exp. Med., 192, F21-F25.
Connolly, P. F., Jäger, R., and Fearnhead, H. O. (2014) New roles for old enzymes: killer caspases as the engine of cell behavior changes, Front. Physiol., 5, 149, https://doi.org/10.3389/fphys.2014.00149.
Radi, E., Formichi, P., Battisti, C., and Federico, A. (2014) Apoptosis and oxidative stress in neurodegenerative diseases, J. Alzheimer’s Dis., 42, S125-S152, https://doi.org/10.3233/JAD-132738.
Chen, C., Zhou, F., Zeng, L., Jiang, Z., and Hu, Z. (2019) Methylene blue offers neuroprotection after intracerebral hemorrhage in rats through the PI3K/Akt/GSK3β signaling pathway, J. Cell Physiol., 234, 5304-5318, https://doi.org/10.1002/jcp.27339.
Abdel-Salam, O., Omara, E., Youness, E., Khadrawy, Y., Mohammed, N., et al. (2014) Rotenone-induced nigrostriatal toxicity is reduced by methylene blue, J. Neurorestoratol., 2, 65-80, https://doi.org/10.2147/JN.S49207.
Lee, K. K., and Boelsterli, U. A. (2014) Bypassing the compromised mitochondrial electron transport with methylene blue alleviates efavirenz/isoniazid-induced oxidant stress and mitochondria-mediated cell death in mouse hepatocytes, Redox Biol., 2, 599-609, https://doi.org/10.1016/j.redox.2014.03.003.
Abdelkader, N. F., Farid, H. A., Youness, E. R., Abdel-Salam, O., and Zaki, H. F. (2020) The role of KATP channel blockade and activation in the protection against neurodegeneration in the rotenone model of Parkinson’s disease, Life Sci., 257, 118070, https://doi.org/10.1016/j.lfs.2020.118070.
Pakavathkumar, P., Sharma, G., Kaushal, V., Foveau, B., and LeBlanc, A. C. (2015) Methylene blue inhibits caspases by oxidation of the catalytic cysteine, Sci. Rep., 5, 13730, https://doi.org/10.1038/srep13730.
Zhou, L., Flores, J., Noël, A., Beauchet, O., Sjöström, P. J., et al. (2019) Methylene blue inhibits Caspase-6 activity, and reverses Caspase-6-induced cognitive impairment and neuroinflammation in aged mice, Acta Neuropathol. Commun., 7, 210, https://doi.org/10.1186/s40478-019-0856-6.
Ahn, H., Kang, S. G., Yoon, S. I., Ko, H. J., Kim, P. H., et al. (2017) Methylene blue inhibits NLRP3, NLRC4, AIM2, and non-canonical inflammasome activation, Sci. Rep., 7, 12409, https://doi.org/10.1038/s41598-017-12635-6.
Amor, S., Puentes, F., Baker, D., and van der Valk, P. (2010) Inflammation in neurodegenerative diseases, Immunology, 129, 154-169, https://doi.org/10.1111/j.1365-2567.2009.03225.x.
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., et al. (2017) Inflammatory responses and inflammation-associated diseases in organs, Oncotarget, 9, 7204-7218, https://doi.org/10.18632/oncotarget.23208.
Zelová, H., and Hošek, J. (2013) TNF-α signalling and inflammation: interactions between old acquaintances, Inflamm. Res., 62, 641-651, https://doi.org/10.1007/s00011-013-0633-0.
Monaco, C., Andreakos, E., Kiriakidis, S., Mauri, C., Bicknell, C., et al. (2004) Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis, Proc. Natl. Acad. Sci. USA, 101, 5634-5639, https://doi.org/10.1073/pnas.0401060101.
Liu, J. J., Lu, L., Hu, F. Q., Yuan, H., Xu, Q., et al. (2018) Methylene blue attenuates renal ischemia-reperfusion injury by negative regulation of NLRP3 signaling pathway, Eur. Rev. Med. Pharmacol. Sci., 22, 2847-2853, https://doi.org/10.26355/eurrev_201805_14986.
Zheng, J., and Li, Q. (2019) Methylene blue regulates inflammatory response in osteoarthritis by noncoding long chain RNA CILinc02, J. Cell Biochem., 120, 3331-3338, https://doi.org/10.1002/jcb.27602.
Wang, S. W., and Sun, Y. M. (2014) The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (review), Int. J. Oncol., 44, 1032-1040, https://doi.org/10.3892/ijo.2014.2259.
Lobo-Silva, D., Carriche, G. M., Castro, A. G., Roque, S., and Saraiva, M. (2016) Balancing the immune response in the brain: IL-10 and its regulation, J. Neuroinflamm., 13, 297, https://doi.org/10.1186/s12974-016-0763-8.
Riley, J. K., Takeda, K., Akira, S., and Schreiber, R. D. (1999) Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action, J. Biol. Chem., 274, 16513-16521, https://doi.org/10.1074/jbc.274.23.16513.
Shih, J. C., Chen, K., and Ridd, M. J. (1999) Role of MAO A and B in neurotransmitter metabolism and behavior, Pol. J. Pharmacol., 51, 25-29.
Godar, S. C., Bortolato, M., Frau, R., Dousti, M., Chen, K., et al. (2011) Maladaptive defensive behaviours in monoamine oxidase A-deficient mice, Int. J. Neuropsychopharmacol., 14, 1195-1207, https://doi.org/10.1017/S1461145710001483.
Watson, C., Bates, J., and Franczak, R. (2009) Serotonin regulation by astrocytes, FASEB J., 23, 790.2, https://doi.org/10.1096/fasebj.23.1_supplement.790.2.
Paudel, P., Park, S. E., Seong, S. H., Jung, H. A., and Choi, J. S. (2019) Novel Diels-Alder type adducts from morus alba root bark targeting human monoamine oxidase and dopaminergic receptors for the management of neurodegenerative diseases, Int. J. Mol. Sci., 20, 6232, https://doi.org/10.3390/ijms20246232.
Ordway, G. A., Farley, J. T., Dilley, G. E., Overholser, J. C., Meltzer, H. Y., et al. (1999) Quantitative distribution of monoamine oxidase A in brainstem monoamine nuclei is normal in major depression, Brain Res., 847, 71-79, https://doi.org/10.1016/s0006-8993(99)02043-0.
Aeschlimann, C., Cerny, T., and Küpfer, A. (1996) Inhibition of (mono)amine oxidase activity and prevention of ifosfamide encephalopathy by methylene blue, Drug Metabol. Dispos., 24, 1336-1339.
Ramsay, R. R., Dunford, C., and Gillman, P. K. (2007) Methylene blue and serotonin toxicity: inhibition of monoamine oxidase A (MAO A) confirms a theoretical prediction, Br. J. Pharmacol., 152, 946-951, https://doi.org/10.1038/sj.bjp.0707430.
Harvey, B. H., Duvenhage, I., Viljoen, F., Scheepers, N., Malan, S. F., et al. (2010) Role of monoamine oxidase, nitric oxide synthase and regional brain monoamines in the antidepressant-like effects of methylene blue and selected structural analogues, Biochem. Pharmacol., 80, 1580-1591, https://doi.org/10.1016/j.bcp.2010.07.037.
Delport, A., Harvey, B. H., Petzer, A., and Petzer, J. P. (2014) Azure B and a synthetic structural analogue of methylene blue, ethylthioninium chloride, present with antidepressant-like properties, Life Sci., 117, 56-66, https://doi.org/10.1016/j.lfs.2014.10.005.
Petzer, A., Harvey, B. H., Wegener, G., and Petzer, J. P. (2012) Azure B, a metabolite of methylene blue, is a high-potency, reversible inhibitor of monoamine oxidase, Toxicol. Appl. Pharmacol., 258, 403-409, https://doi.org/10.1016/j.taap.2011.12.005.
Reis, P. A., Gonçalves de Albuquerque, C. F., Maron-Gutierrez, T., Silva, A. R, and de Castro Faria Neto, H. C. (2017) Role of nitric oxide synthase in the function of the central nervous system under normal and infectious conditions, Nitric Oxide Synthase, IntechOpen, https://doi.org/10.5772/67816.
Czapski, G. A., Cakala, M., Chalimoniuk, M., Gajkowska, B., and Strosznajder, J. B. (2007) Role of nitric oxide in the brain during lipopolysaccharide-evoked systemic inflammation, J. Neurosci. Res., 85, 1694-1703, https://doi.org/10.1002/jnr.21294.
Zhou, X. Y., Zhang, F., Ying, C. J., Chen, J., Chen, L., et al. (2017) Inhibition of iNOS alleviates cognitive deficits and depression in diabetic mice through downregulating the NO/sGC/cGMP/PKG signal pathway, Behav. Brain Res., 322, 70-82, https://doi.org/10.1016/j.bbr.2016.12.046.
Chen, H. J., Spiers, J. G., Sernia, C., and Lavidis, N. A. (2015) Response of the nitrergic system to activation of the neuroendocrine stress axis, Front. Neurosci., 9, 3, https://doi.org/10.3389/fnins.2015.00003.
Zhou, Q. G., Zhu, L. J., Chen, C., Wu, H. Y., Luo, C. X., et al. (2011) Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor, J. Neurosci., 31, 7579-7590, https://doi.org/10.1523/JNEUROSCI.0004-11.2011.
Gragnoli, C. (2014) Hypothesis of the neuroendocrine cortisol pathway gene role in the comorbidity of depression, type 2 diabetes, and metabolic syndrome, Appl. Clin. Genet., 7, 43-53, https://doi.org/10.2147/TACG.S39993.
Mayer, B., Brunner, F., and Schmidt, K. (1993) Inhibition of nitric oxide synthesis by methylene blue, Biochem. Pharmacol., 45, 367-374, https://doi.org/10.1016/0006-2952(93)90072-5.
Ignarro, L. J., Burke, T. M., Wood, K. S., Wolin, M. S., and Kadowitz, P. J. (1984) Association between cyclic GMP accumulation and acetylcholine-elicited relaxation of bovine intrapulmonary artery, J. Pharmacol. Exp. Ther., 228, 682-690.
Luo, D., Das, S., and Vincent, S. R. (1995) Effects of methylene blue and LY83583 on neuronal nitric oxide synthase and NADPH-diaphorase, Eur. J. Pharmacol., 290, 247-251, https://doi.org/10.1016/0922-4106(95)00084-4.
Volke, V., Wegener, G., Vasar, E., and Rosenberg, R. (1999) Methylene blue inhibits hippocampal nitric oxide synthase activity in vivo, Brain Res., 826, 303-305, https://doi.org/10.1016/s0006-8993(99)01253-6.
Deutsch, S., Rosse, R., Paul, S., Tomasino, V., Koetzner, L., et al. (1996) 7-Nitroindazole and methylene blue, inhibitors of neuronal nitric oxide synthase and NO-stimulated guanylate cyclase, block MK-801-elicited behaviors in mice, Neuropsychopharmacology, 15, 37-43, https://doi.org/10.1016/0893-133X(95)00153-5.
Lomniczi, A., Cebral, E., Canteros, G., McCann, S. M., and Rettori, V. (2000) Methylene blue inhibits the increase of inducible nitric oxide synthase activity induced by stress and lipopolysaccharide in the medial basal hypothalamus of rats, Neuroimmunomodulation, 8, 122-127, https://doi.org/10.1159/000054271.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation under the State assignment for universities in the field of scientific activity for 2020-2022 (project FZGU-2020-0044), scholarships of the President of the Russian Federation for young scientists and graduate students (SP-2802.2021.4), and a grant from the President of the Russian Federation for state support of young Russian scientists and state support for leading scientific schools (NSh-1375.2022.5).
Author information
Authors and Affiliations
Contributions
Artem P. Gureev – writing the text, Irina S. Sadovnikova – search and annotation of articles, preparation of drawings, Vasily N. Popov – concept and administration.
Corresponding author
Ethics declarations
The authors declare no conflicts of interests in financial or any other sphere. This article does not contain a description of any research involving humans or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Gureev, A.P., Sadovnikova, I.S. & Popov, V.N. Molecular Mechanisms of the Neuroprotective Effect of Methylene Blue. Biochemistry Moscow 87, 940–956 (2022). https://doi.org/10.1134/S0006297922090073
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297922090073