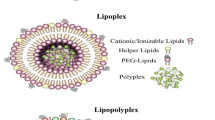
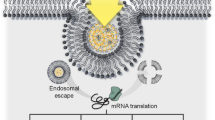
Abstract
In this review we discuss features of mRNA synthesis and modifications used to minimize immune response and prolong efficiency of the translation process in vivo. Considerable attention is given to the use of liposomes and nanoparticles containing lipids and polymers for the mRNA delivery. Finally we briefly discuss mRNAs which are currently in the clinical trials for cancer immunotherapy, vaccination against infectious diseases, and replacement therapy.
Similar content being viewed by others
Abbreviations
- CRISPR-Cas9:
-
system of clustered regularly interspaced short palindromic repeats and Cas9 nuclease
- dsRNA:
-
double stranded RNA
- FDA:
-
Food and Drug Administration (USA)
- HIV:
-
human immunodeficiency virus
- LNP:
-
lipid nanoparticles
- mRNA:
-
messenger RNA
- NA:
-
nucleic acids
- ORF:
-
open reading frame
- PEG:
-
polyethylene glycol
- siRNA:
-
small interfering RNA
- TALEN:
-
transcription activator-like effector nuclease
- TLRs:
-
Toll-like receptors
- UTR:
-
untranslated region
- ZFN:
-
zinc finger nuclease
References
Zamecnik, P. C., and Stephenson, M. L. (1978) Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide, Proc. Natl. Acad. Sci. USA, 75, 280–284.
Stephenson, M. L., and Zamecnik, P. C. (1978) Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide, Proc. Natl. Acad. Sci. USA, 75, 285–288.
Bennett, C. F., and Swayze, E. E. (2010) RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform, Annu. Rev. Pharmacol. Toxicol., 50, 259–293.
Lundin, K. E., Gissberg, O., and Smith, C. I. (2015) Oligonucleotide therapies: the past and the present, Hum. Gene Ther., 26, 475–485.
Dobrovolskaia, M. A., and McNeil, S. E. (2015) Immunological and hematological toxicities challenging clinical translation of nucleic acid-based therapeutics, Expert Opin. Biol. Ther., 15, 1023–1048.
Bobbin, M. L., and Rossi, J. J. (2016) RNA interference (RNAi)-based therapeutics: delivering on the promise? Annu. Rev. Pharmacol. Toxicol., 56, 103–122.
Prakash, V., Moore, M., and Yanez-Munoz, R. J. (2016) Current progress in therapeutic gene editing for monogenic diseases, Mol. Ther., 24, 465–474.
LaFountaine, J. S., Fathe, K., and Smyth, H. D. (2015) Delivery and therapeutic applications of gene editing technologies ZFNs, TALENs, and CRISPR/Cas9, Int. J. Pharm., 494, 180–194.
Maeder, M. L., and Gersbach, C. A. (2016) Genome-editing technologies for gene and cell therapy, Mol. Ther., 24, 430–446.
Lander, E. S. (2016) The heroes of CRISPR, Cell, 164, 18–28.
Lee, C. M., Cradick, T. J., Fine, E. J., and Bao, G. (2016) Nuclease target site selection for maximizing on-target activity and minimizing off-target effects in genome editing, Mol. Ther., 24, 475–487.
Yin, H., Xue, W., Chen, S., Bogorad, R. L., Benedetti, E., Grompe, M., Koteliansky, V., Sharp, P. A., Jacks, T., and Anderson, D. G. (2014) Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype, Nat. Biotechnol., 32, 551–553.
Yin, H., Song, C. Q., Dorkin, J. R., Zhu, L. J., Li, Y., Wu, Q., Park, A., Yang, J., Suresh, S., Bizhanova, A., Gupta, A., Bolukbasi, M. F., Walsh, S., Bogorad, R. L., Gao, G., Weng, Z., Dong, Y., Koteliansky, V., Wolfe, S. A., Langer, R., Xue, W., and Anderson, D. G. (2016) Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo, Nat. Biotechnol., 34, 328–333.
Prazeres, D. M., and Monteiro, G. A. (2014) Plasmid biopharmaceuticals, Microbiol. Spectr., 2, doi: 10.1128/microbiolspec.PLAS-0022-2014.
Meunier, M., Chemaly, M., and Dory, D. (2016) DNA vaccination of poultry: the current status in 2015, Vaccine, 34, 202–211.
Fewell, J. G., MacLaughlin, F., Mehta, V., Gondo, M., Nicol, F., Wilson, E., and Smith, L. C. (2001) Gene therapy for the treatment of hemophilia B using PINC-formulated plasmid delivered to muscle with electroporation, Mol. Ther., 3, 574–583.
Nikol, S., Baumgartner, I., Van Belle, E., Diehm, C., Visona, A., Capogrossi, M. C., Ferreira-Maldent, N., Gallino, A., Wyatt, M. G., Wijesinghe, L. D., Fusari, M., Stephan, D., Emmerich, J., Pompilio, G., Vermassen, F., Pham, E., Grek, V., Coleman, M., and Meyer, F. (2008) Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia, Mol. Ther., 16, 972–978.
Sahin, U., Kariko, K., and Ureci, O. (2014) mRNA-based therapeutics–developing a new class of drugs, Nat. Rev. Drug Discov., 13, 759–780.
Matsui, A., Uchida, S., Ishii, T., Itaka, K., and Kataoka, K. (2015) Messenger RNA-based therapeutics for the treatment of apoptosis-associated diseases, Sci. Rep., 5, 15810.
Warren, L., Manos, P. D., Ahfeldt, T., Loh, Y. H., Li, H., Lau, F., Ebina, W., Mandal, P. K., Smith, Z. D., Meissner, A., Daley, G. Q., Brack, A. S., Collins, J. J., Cowan, C., Schlaeger, T. M., and Rossi, D. J. (2010) Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA, Cell Stem Cell, 7, 618–630.
Uchida, S., Kataoka, K., and Itaka, K. (2015) Screening of mRNA chemical modification to maximize protein expression with reduced immunogenicity, Pharmaceutics, 7, 137–151.
Mahiny, A. J., Dewerth, A., Mays, L. E., Alkhaled, M., Mothes, B., Malaeksefat, E., Loretz, B., Rottenberger, J., Brosch, D. M., Reautschnig, P., Surapolchai, P., Zeyer, F., Schams, A., Carevic, M., Bakele, M., Griese, M., Schwab, M., Nurnberg, B., Beer-Hammer, S., Handgretinger, R., Hartl, D., Lehr, C. M., and Kormann, M. S. (2015) In vivo genome editing using nuclease-encoding mRNA corrects SP-B deficiency, Nat. Biotechnol., 33, 584–586.
Mehier-Humbert, S., and Guy, R. H. (2005) Physical methods for gene transfer: improving the kinetics of gene delivery into cells, Adv. Drug Deliv. Rev., 57, 733–753.
Zhang, X., and Godbey, W. T. (2006) Viral vectors for gene delivery in tissue engineering, Adv. Drug Deliv. Rev., 58, 515–534.
Kotterman, M. A., and Schaffer, D. V. (2014) Engineering adeno-associated viruses for clinical gene therapy, Nat. Rev. Genet., 15, 445–451.
Andreev, D. E., Terenin, I. M., Dmitriev, S. E., and Shatsky, I. N. (2016) Pros and cons of pDNA and mRNA transfection to study mRNA translation in mammalian cells, Gene, 578, 1–6.
Gallie, D. R. (1991) The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency, Genes Dev., 5, 2108–2116.
Kuhn, A. N., Diken, M., Kreiter, S., Selmi, A., Kowalska, J., Jemielity, J., Darzynkiewicz, E., Huber, C., Tureci, O., and Sahin, U. (2010) Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immaturedendritic cells and induce superior immune responses in vivo, Gene Ther., 17, 961–971.
Stepinski, J., Waddell, C., Stolarski, R., Darzynkiewicz, E., and Rhoads, R. E. (2001) Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl(3'O-methyl)GpppG and 7-methyl(3'-deoxy)GpppG, RNA, 7, 1486–1495.
Grudzien-Nogalska, E., Jemielity, J., Kowalska, J., Darzynkiewicz, E., and Rhoads, R. E. (2007) Phosphorothioate cap analogs stabilize mRNA and increase translational efficiency in mammalian cells, RNA, 13, 1745–1755.
Loo, Y. M., Fornek, J., Crochet, N., Bajwa, G., Perwitasari, O., Martinez-Sobrido, L., Akira, S., Gill, M. A., Garcia-Sastre, A., Katze, M. G., and Gale, M. (2008) Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity, J. Virol., 82, 335–345.
Pichlmair, A., Schulz, O., Tan, C. P., Rehwinkel, J., Kato, H., Takeuchi, O., Akira, S., Way, M., Schiavo, G., and Reise Sousa, C. (2009) Activation of MDA5 requires higherorder RNA structures generated during virus infection, J. Virol., 83, 10761–10769.
Lu, C., Xu, H., Ranjith-Kumar, C. T., Brooks, M. T., Hou, T. Y., Hu, F., Herr, A. B., Strong, R. K., Kao, C. C., and Li, P. (2010) The structural basis of 5'-triphosphate doublestranded RNA recognition by RIG-I C-terminal domain, Structure, 18, 1032–1043.
Yoneyama, M., and Fujita, T. (2010) Recognition of viral nucleic acids in innate immunity, Rev. Med. Virol., 20, 4–22.
Sen, G. C., and Sarkar, S. N. (2007) The interferon-stimulated genes: targets of direct signaling by interferons, double-stranded RNA, and viruses, Curr. Top. Microbiol. Immunol., 316, 233–250.
Andries, O., De Filette, M., De Smedt, S. C., Demeester, J., Van Poucke, M., Peelman, L., and Sanders, N. N. (2013) Innate immune response and programmed cell death following carrier-mediated delivery of unmodified mRNA to respiratory cells, J. Control. Release, 167, 157–166.
Kanneganti, T. D., Body-Malapel, M., Amer, A., Park, J. H., Whitfield, J., Franchi, L., Taraporewala, Z. F., Miller, D., Patton, J. T., Inohara, N., and Nunez, G. (2006) Critical role for cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA, J. Biol. Chem., 281, 36560–36568.
Nallagatla, S. R., Hwang, J., Toroney, R., Zheng, X., Cameron, C. E., and Bevilacqua, P. C. (2007) 5'Triphosphate-dependent activation of PKR by RNAs with short stem-loops, Science, 318, 1455–1458.
Pardi, N., Muramatsu, H., Weissman, D., and Kariko, K. (2013) In vitro transcription of long RNA containing modified nucleosides, Methods Mol. Biol., 969, 29–42.
Brawerman, G. (1981) The role of the poly(A) sequence in mammalian messenger RNA, CRC Crit. Rev. Biochem., 10, 1–38.
Koski, G. K., Kariko, K., Xu, S., Weissman, D., Cohen, P. A., and Czerniecki, B. J. (2004) Cutting edge: innate immune system discriminates between RNA containing bacterial versus eukaryotic structural features that prime for high-level IL-12 secretion by dendritic cells, J. Immunol., 172, 3989–3993.
Caput, D., Beutler, B., Hartog, K., Thayer, R., BrownShimer, S., and Cerami, A. (1986) Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators, Proc. Natl. Acad. Sci. USA, 83, 1670–1674.
Holtkamp, S. (2006) Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells, Blood, 108, 4009–4017.
Kariko, K., Kuo, A., and Barnathan, E. (1999) Overexpression of urokinase receptor in mammalian cells following administration of the in vitro transcribed encoding mRNA, Gene Ther., 6, 1092–1100.
Diebold, S. S., Massacrier, C., Akira, S., Paturel, C., Morel, Y., and Reise Sousa, C. (2006) Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides, Eur. J. Immunol., 36, 3256–3267.
Alexopoulou, L., Holt, A. C., Medzhitov, R., and Flavell, R. A. (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3, Nature, 413, 732–738.
Kariko, K., Buckstein, M., Ni, H., and Weissman, D. (2005) Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA, Immunity, 23, 165–175.
Nallagatla, S. R., and Bevilacqua, P. C. (2008) Nucleoside modifications modulate activation of the protein kinase PKR in an RNA structure-specific manner, RNA, 14, 1201–1213.
Kariko, K., Muramatsu, H., Welsh, F. A., Ludwig, J., Kato, H., Akira, S., and Weissman, D. (2008) Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability, Mol. Ther., 16, 1833–1840.
Li, B., Luo, X., and Dong, Y. (2016) Effects of chemically modified messenger RNA on protein expression, Bioconj. Chem., 27, 849–853.
Kormann, M. S., Hasenpusch, G., Aneja, M. K., Nica, G., Flemmer, A. W., Herber-Jonat, S., Huppmann, M., Mays, L. E., Illenyi, M., Schams, A., Griese, M., Bittmann, I., Handgretinger, R., Hartl, D., Rosenecker, J., and Rudolph, C. (2011) Expression of therapeutic proteins after delivery of chemically modified mRNA in mice, Nat. Biotechnol., 29, 154–157.
Wang, X., Zhao, B. S., Roundtree, I. A., Lu, Z., Han, D., Ma, H., Weng, X., Chen, K., Shi, H., and He, C. (2015) N6-methyladenosine modulates messenger RNA translation efficiency, Cell, 161, 1388–1399.
Kariko, K., Muramatsu, H., Ludwig, J., and Weissman, D. (2011) Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA, Nucleic Acids Res., 39, e142.
Rittig, S. M., Haentschel, M., Weimer, K. J., Heine, A., Muller, M. R., Brugger, W., Horger, M. S., Maksimovic, O., Stenzl, A., Hoerr, I., Rammensee, H. G., Holderried, T. A., Kanz, L., Pascolo, S., and Brossart, P. (2011) Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients, Mol. Ther., 19, 990–999.
Deering, R. P., Kommareddy, S., Ulmer, J. B., Brito, L. A., and Geall, A. J. (2014) Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines, Expert Opin. Drug Deliv., 11, 885–899.
Brito, L. A., Kommareddy, S., Maione, D., Uematsu, Y., Giovani, C., Berlanda Scorza, F., Otten, G. R., Yu, D., Mandl, C. W., Mason, P. W., Dormitzer, P. R., Ulmer, J. B., and Geall, A. J. (2015) Self-amplifying mRNA vaccines, Adv. Genet., 89, 179–233.
Loomis, K. H., Kirschman, J. L., Bhosle, S., Bellamkonda, R. V., and Santangelo, P. J. (2016) Strategies for modulating innate immune activation and protein production of in vitro transcribed mRNAs, J. Mat. Chem. B, 4, 1619–1632.
Islam, M. A., Reesor, E. K. G., Xu, Y., Zope, H. R., Zetter, B. R., and Shia, J. (2015) Biomaterials for mRNA delivery, Biomater. Sci., 3, 1519–1533.
Kauffman, K. J., Webber, M. J., and Anderson, D. G. (2015) Materials for non-viral intracellular delivery of messenger RNA therapeutics, J. Control. Release, 3 pii: S0168-3659(15)30283-2.
Van Lint, S., Goyvaerts, C., Maenhout, S., Goethals, L., Disy, A., Benteyn, D., Pen, J., Bonehill, A., Heirman, C., Breckpot, K., and Thielemans, K. (2012) Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy, Cancer Res., 72, 1661–1671.
Amos, H. (1961) Protamine enhancement of RNA uptake by cultured chick cells, Biochem. Biophys. Res. Commun., 5, 1–4.
Choi, Y. S., Lee, J. Y., Suh, J. S., Kwon, Y. M., Lee, S. J., Chung, J. K., Lee, D. S., Yang, V. C., Chung, C. P., and Park, Y. J. (2010) The systemic delivery of siRNAs by a cell penetrating peptide, low molecular weight protamine, Biomaterials, 31, 1429–1443.
Kallen, K. J., Heidenreich, R., Schnee, M., Petsch, B., Schlake, T., Thess, A., Baumhof, P., Scheel, B., Koch, S. D., and Fotin-Mleczek, M. (2013) A novel, disruptive vaccination technology: self-adjuvanted RNActive® vaccines, Hum. Vaccin. Immunother., 9, 2263–2276.
Brito, L. A., Chan, M., Shaw, C. A., Hekele, A., Carsillo, T., Schaefer, M., Archer, J., Seubert, A., Otten, G. R., Beard, C. W., Dey, A. K., Lilja, A., Valiante, N. M., Mason, P. W., Mandl, C. W., Barnett, S. W., Dormitzer, P. R., Ulmer, J. B., Singh, M., O’Hagan, D. T., and Geall, A. J. (2014) A cationic nanoemulsion for the delivery of nextgeneration RNA vaccines, Mol. Ther., 22, 2118–2129.
Safinya, C. R. (2001) Structures of lipid–DNA complexes: supramolecular assembly and gene delivery, Curr. Opin. Struct. Biol., 11, 440–448.
Mockey, M., Bourseau, E., Chandrashekhar, V., Chaudhuri, A., Lafosse, S., Le Cam, E., Quesniaux, V. F., Ryffel, B., Pichon, C., and Midoux, P. (2007) mRNAbased cancer vaccine: prevention of B16 melanoma progression and metastasis by systemic injection of MART1 mRNA histidylated lipopolyplexes, Cancer Gene Ther., 14, 802–814.
Hess, P. R., Boczkowski, D., Nair, S. K., Snyder, D., and Gilboa, E. (2006) Vaccination with mRNAs encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes CTL responses, but is insufficient to overcome tolerance to a model tumor/self antigen, Cancer Immunol. Immunother., 55, 672–683.
Pollard, C., Rejman, J., De Haes, W., Verrier, B., Van Gulck, E., Naessens, T., De Smedt, S., Bogaert, P., Grooten, J., Vanham, G., and De Koker, S. (2013) Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines, Mol. Ther., 21, 251–259.
Geall, A. J., Verma, A., Otten, G. R., Shaw, C. A., Hekele, A., Banerjee, K., Cu, Y., Beard, C. W., Brito, L. A., Krucker, T., O’Hagan, D. T., Singh, M., Mason, P. W., Valiante, N. M., Dormitzer, P. R., Barnett, S. W., Rappuoli, R., Ulmer, J. B., and Mandl, C. W. (2012) Nonviral delivery of self-amplifying RNA vaccines, Proc. Natl. Acad. Sci. USA, 109, 14604–14609.
Cheng, X., and Lee, R. J. (2016) The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery, Adv. Drug Deliv. Rev., 99, 129–137.
Tanaka, H., Sato, Y., Harashima, H., and Akita, H. (2016) Cellular environment-responsive nanomaterials for use in gene and siRNA delivery: molecular design for biomembrane destabilization and intracellular collapse, Expert Opin. Drug Deliv., 21, 1–13.
Maier, M. A., Jayaraman, M., Matsuda, S., Liu, J., Barros, S., Querbes, W., Tam, Y. K., Ansell, S. M., Kumar, V., Qin, J., Zhang, X., Wang, Q., Panesar, S., Hutabarat, R., Carioto, M., Hettinger, J., Kandasamy, P., Butler, D., Rajeev, K. G., Pang, B., Charisse, K., Fitzgerald, K., Mui, B. L., Du, X., Cullis, P., Madden, T. D., Hope, M. J., Manoharan, M., and Akinc, A. (2013) Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics, Mol. Ther., 21, 1570–1578.
Pardi, N., Tuyishime, S., Muramatsu, H., Kariko, K., Mui, B. L., Tam, Y. K., Madden, T. D., Hope, M. J., and Weissman, D. (2015) Expression kinetics of nucleosidemodified mRNA delivered in lipid nanoparticles to mice by various routes, J. Control. Release, 217, 345–351.
Naseri, N., Valizadeh, H., and Zakeri-Milani, P. (2015) Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application, Adv. Pharm. Bull., 5, 305–313.
Midoux, P., and Pichon, C. (2015) Lipid-based mRNA vaccine delivery systems, Expert Rev. Vaccines, 14, 221–234.
Warashina, S., Nakamura, T., Sato, Y., Fujiwara, Y., Hyodo, M., Hatakeyama, H., and Harashima, H. (2016) A lipid nanoparticle for the efficient delivery of siRNA to dendritic cells, J. Control. Release, 225, 183–191.
Wang, Y., Rajala, A., and Rajala, R. V. (2015) Lipid nanoparticles for ocular gene delivery, J. Funct. Biomater., 6, 379–394.
Kim, Y. D., Park, T. E., Singh, B., Maharjan, S., Choi, Y. J., Choung, P. H., Arote, R. B., and Cho, C. S. (2015) Nanoparticle-mediated delivery of siRNA for effective lung cancer therapy, Nanomedicine, 10, 1165–1188.
Li, B., Luo, X., Deng, B., Wang, J., McComb, D. W., Shi, Y., Gaensler, K. M., Tan, X., Dunn, A. L., Kerlin, B. A., and Dong, Y. (2015) An orthogonal array optimization of lipid-like nanoparticles for mRNA delivery in vivo, Nano Lett., 15, 8099–8107.
Li, B., Luo, X., Deng, B., Giancola, J. B., McComb, D. W., Schmittgen, T. D., and Dong, Y. (2016) Effects of local structural transformation of lipid-like compounds on delivery of messenger RNA, Sci. Rep., 6, 22137.
Kauffman, K. J., Dorkin, J. R., Yang, J. H., Heartlein, M. W., DeRosa, F., Mir, F. F., Fenton, O. S., and Anderson, D. G. (2015) Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs, Nano Lett., 15, 7300–7306.
Fenton, O. S., Kauffman, K. J., McClellan, R. L., Appel, E. A., Dorkin, J. R., Tibbitt, M. W., Heartlein, M. W., DeRosa, F., Langer, R., and Anderson, D. G. (2016) Bioinspired alkenyl amino alcohol ionizable lipid materials for highly potent in vivo mRNA delivery, Adv. Mater., 28, 2939–2943.
Akinc, A., Querbes, W., De, S., Qin, J., FrankKamenetsky, M., Jayaprakash, K. N., Jayaraman, M., Rajeev, K. G., Cantley, W. L., Dorkin, J. R., Butler, J. S., Qin, L., Racie, T., Sprague, A., Fava, E., Zeigerer, A., Hope, M. J., Zerial, M., Sah, D. W., Fitzgerald, K., Tracy, M. A., Manoharan, M., Koteliansky, V., Fougerolles, A., and Maier, M. A. (2010) Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms, Mol. Ther., 18, 1357–1364.
Kumar, V., Qin, J., Jiang, Y., Duncan, R. G., Brigham, B., Fishman, S., Nair, J. K., Akinc, A., Barros, S. A., and Kasperkovitz, P. V. (2014) Shielding of lipid nanoparticles for siRNA delivery: impact on physicochemical properties, cytokine induction, and efficacy, Mol. Ther. Nucleic Acids, 3, e210.
Markov, O. V., Mironova, N. L., Shmendel, E. V., Serikov, R. N., Morozova, N. G., Maslov, M. A., Vlassov, V. V., and Zenkova, M. A. (2015) Multicomponent mannose-containing liposomes efficiently deliver RNA in murine immature dendritic cells and provide productive anti-tumour response in murine melanoma model, J. Control. Release, 213, 45–56.
Crowley, S. T., Poliskey, J. A., Baumhover, N. J., and Rice, K. G. (2015) Efficient expression of stabilized mRNA PEG-peptide polyplexes in liver, Gene Ther., 22, 993–999.
Lachelt, U., and Wagner, E. (2015) Nucleic acid therapeutics using polyplexes: a journey of 50 years (and beyond), Chem. Rev., 115, 11043–11078.
Rozenkrants, A. A., and Sobolev, A. S. (2015) Polyethylenimine polyplex nanoparticles and their behavior in cells and body tissues, Izv. Akad. Nauk Ser. Khim., 2749-2755.
Rejman, J., Tavernier, G., Bavarsad, N., Demeester, J., and De Smedt, S. C. (2010) mRNA transfection of cervical carcinoma and mesenchymal stem cells mediated by cationic carriers, J. Control. Release, 147, 385–391.
Demoulins, T., Milona, P., Englezou, P. C., Ebensen, T., Schulze, K., Suter, R., Pichon, C., Midoux, P., Guzman, C. A., Ruggli, N., and McCullough, K. C. (2015) Polyethylenimine-based polyplex delivery of self-replicating RNA vaccines, Nanomedicine, 12, 711–722.
Bettinger, T., Carlisle, R. C., Read, M. L., Ogris, M., and Seymour, L. W. (2001) Peptide-mediated RNA delivery: a novel approach for enhanced transfection of primary and post-mitotic cells, Nucleic Acids Res., 29, 3882–3891.
Su, X., Fricke, J., Kavanagh, D. G., and Irvine, D. J. (2011) In vitro and in vivo mRNA delivery using lipidenveloped pH-responsive polymer nanoparticles, Mol. Pharm., 8, 774–787.
Mockey, M., Bourseau, E., Chandrashekhar, V., Chaudhuri, A., Lafosse, S., Le Cam, E., Quesniaux, V. F., Ryffel, B., Pichon, C., and Midoux, P. (2007) mRNAbased cancer vaccine: prevention of B16 melanoma progression and metastasis by systemic injection of MART1 mRNA histidylated lipopolyplexes, Cancer Gene Ther., 14, 802–814.
Uchida, H., Itaka, K., Nomoto, T., Ishii, T., Suma, T., Ikegami, M., Miyata, K., Oba, M., Nishiyama, N., and Kataoka, K. (2014) Modulated protonation of side chain aminoethylene repeats in N-substituted polyaspartamides promotes mRNA transfection, J. Am. Chem. Soc., 136, 12396–12405.
Uchida, H., Itaka, K., Uchida, S., Ishii, T., Suma, T., Miyata, K., Oba, M., Nishiyama, N., and Kataoka, K. (2016) Synthetic polyamines to regulate mRNA translation through the preservative binding of eukaryotic initiation factor 4E to the cap structure, J. Am. Chem. Soc., 138, 1478–1481.
Phua, K. K., Leong, K. W., and Nair, S. K. (2013) Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format, J. Control. Release, 166, 227–233.
Uchida, S., Kinoh, H., Ishii, T., Matsui, A., Tockary, T. A., Takeda, K. M., Uchida, H., Osada, K., Itaka, K., and Kataoka, K. (2016) Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety, Biomaterials, 82, 221–228.
Perche, F., Benvegnu, T., Berchel, M., Lebegue, L., Pichon, C., Jaffres, P. A., and Midoux, P. (2011) Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA, Nanomedicine, 7, 445–453.
Perche, F., Gosset, D., Mevel, M., Miramon, M. L., Yaouanc, J. J., Pichon, C., Benvegnu, T., Jaffres, P. A., and Midoux, P. (2011) Selective gene delivery in dendritic cells with mannosylated and histidylated lipopolyplexes, J. Drug Target., 19, 315–325.
Srinivas, R., Karmali, P. P., Pramanik, D., Garu, A., Mahidhar, Y. V., Majeti, B. K., Ramakrishna, S., Srinivas, G., and Chaudhuri, A. (2010) Cationic amphiphile with shikimic acid headgroup shows more systemic promise than its mannosyl analogue as DNA vaccine carrier in dendritic cell based genetic immunization, J. Med. Chem., 53, 1387–1391.
Srinivas, R., Garu, A., Moku, G., Agawane, S. B., and Chaudhuri, A. (2012) A long-lasting dendritic cell DNA vaccination system using lysinylated amphiphiles with mannose-mimicking head-groups, Biomaterials, 33, 6220–6229.
Johler, S. M., Rejman, J., Guan, S., and Rosenecker, J. (2015) Nebulisation of IVT mRNA complexes for intrapulmonary administration, PLoS One, 10, e0137504.
Uchida, S., Itaka, K., Uchida, H., Hayakawa, K., Ogata, T., Ishii, T., Fukushima, S., Osada, K., and Kataoka, K. (2013) In vivo messenger RNA introduction into the central nervous system using polyplex nanomicelle, PLoS One, 8, e56220.
Baba, M., Itaka, K., Kondo, K., Yamasoba, T., and Kataoka, K. (2015) Treatment of neurological disorders by introducing mRNA in vivo using polyplex nanomicelles, J. Control. Release, 201, 41–48.
Weiss, R., Scheiblhofer, S., Roesler, E., Weinberger, E., and Thalhamer, J. (2012) mRNA vaccination as a safe approach for specific protection from type I allergy, Expert Rev. Vaccines, 11, 55–67.
Scheel, B., Teufel, R., Probst, J., Carralot, J. P., Geginat, J., Radsak, M., Jarrossay, D., Wagner, H., Jung, G., Rammensee, H. G., Hoerr, I., and Pascolo, S. (2005) Tolllike receptor-dependent activation of several human blood cell types by protamine-condensed mRNA, Eur. J. Immunol., 35, 1557–1566.
Heiser, A., Coleman, D., Dannull, J., Yancey, D., Maurice, M. A., Lallas, C. D., Dahm, P., Niedzwiecki, D., Gilboa, E., and Vieweg, J. (2002) Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors, J. Clin. Invest., 109, 409–417.
Kubler, H., Scheel, B., Gnad-Vogt, U., Miller, K., Schultze-Seemann, W., Vom Dorp, F., Parmiani, G., Hampel, C., Wedel, S., Trojan, L., Jocham, D., Maurer, T., Rippin, G., Fotin-Mleczek, M., Von der Mulbe, F., Probst, J., Hoerr, I., Kallen, K. J., Lander, T., and Stenzl, A. (2015) Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study, J. Immunother. Cancer, 3, 26.
Su, Z., Dannull, J., Heiser, A., Yancey, D., Pruitt, S., Madden, J., Coleman, D., Niedzwiecki, D., Gilboa, E., and Vieweg, J. (2003) Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells, Cancer Res., 63, 2127–2133.
Dannull, J., Su, Z., Rizzieri, D., Yang, B. K., Coleman, D., Yancey, D., Zhang, A., Dahm, P., Chao, N., Gilboa, E., and Vieweg, J. (2005) Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T-cells, J. Clin. Invest., 115, 3623–3633.
Mu, L. J., Kyte, J. A., Kvalheim, G., Aamdal, S., Dueland, S., Hauser, M., Hammerstad, H., Waehre, H., Raabe, N., and Gaudernack, G. (2005) Immunotherapy with allotumour mRNA-transfected dendritic cells in androgenresistant prostate cancer patients, Br. J. Cancer, 93, 749–756.
Kyte, J. A., Mu, L., Aamdal, S., Kvalheim, G., Dueland, S., Hauser, M., Gullestad, H. P., Ryder, T., Lislerud, K., Hammerstad, H., and Gaudernack, G. (2006) Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA, Cancer Gene Ther., 13, 905–918.
Weide, B., Carralot, J. P., Reese, A., Scheel, B., Eigentler, T. K., Hoerr, I., Rammensee, H. G., Garbe, C., and Pascolo, S. (2008) Results of the first phase I/II clinical vaccination trial with direct injection of mRNA, J. Immunother., 31, 180–188.
Fotin-Mleczek, M., Duchardt, K. M., Lorenz, C., Pfeiffer, R., Ojkic-Zrna, S., Probst, J., and Kallen, K. J. (2011) Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity, J. Immunother., 34, 1–15.
Pascolo, S. (2008) Vaccination with messenger RNA (mRNA), Handb. Exp. Pharmacol., 183, 221–235.
Martinon, F., Krishnan, S., Lenzen, G., Magne, R., Gomard, E., Guillet, J. G., Levy, J. P., and Meulien, P. (1993) Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA, Eur. J. Immunol., 23, 1719–1722.
Petsch, B., Schnee, M., Vogel, A. B., Lange, E., Hoffmann, B., Voss, D., Schlake, T., Thess, A., Kallen, K. J., Stitz, L., and Kramps, T. (2012) Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection, Nat. Biotechnol., 30, 1210–1216.
Brazzoli, M., Magini, D., Bonci, A., Buccato, S., Giovani, C., Kratzer, R., Zurli, V., Mangiavacchi, S., Casini, D., Brito, L. M., De Gregorio, E., Mason, P. W., Ulmer, J. B., Geall, A. J., and Bertholet, S. (2015) Induction of broad-based immunity and protective efficacy by self-amplifying mRNA vaccines encoding influenza virus hemagglutinin, J. Virol., 90, 332–344.
Allard, S. D., De Keersmaecker, B., De Goede, A. L., Verschuren, E. J., Koetsveld, J., Reedijk, M. L., Wylock, C., De Bel, A. V., Vandeloo, J., Pistoor, F., Heirman, C., Beyer, W. E., Eilers, P. H., Corthals, J., Padmos, I., Thielemans, K., Osterhaus, A. D., Lacor, P., Van der Ende, M. E., Aerts, J. L., Van Baalen, C. A., and Gruters, R. A. (2012) A phase I/IIa immunotherapy trial of HIV-1infected patients with Tat, Rev and Nef expressing dendritic cells followed by treatment interruption, Clin. Immunol., 142, 252–268.
Van Gulck, E., Vlieghe, E., Vekemans, M., Van Tendeloo, V. F., Van De Velde, A., Smits, E., Anguille, S., Cools, N., Goossens, H., Mertens, L., De Haes, W., Wong, J., Florence, E., Vanham, G., and Berneman, Z. N. (2012) mRNA-based dendritic cell vaccination induces potent antiviral T-cell responses in HIV-1-infected patients, AIDS, 26, 1–12.
Mays, L. E., Ammon-Treiber, S., Mothes, B., Alkhaled, M., Rottenberger, J., Muller-Hermelink, E. S., Grimm, M., Mezger, M., Beer-Hammer, S., Von Stebut, E., Rieber, N., Nurnberg, B., Schwab, M., Handgretinger, R., Idzko, M., Hartl, D., and Kormann, M. S. (2013) Modified Foxp3 mRNA protects against asthma through an IL-10dependent mechanism, J. Clin. Invest., 123, 1216–1228.
Lui, K. O., Zangi, L., and Chien, K. R. (2014) Cardiovascular regenerative therapeutics via synthetic paracrine factor modified mRNA, Stem Cell Res., 13, 693–704.
Zangi, L., Lui, K. O., Von Gise, A., Ma, Q., Ebina, W., Ptaszek, L. M., Spater, D., Xu, H., Tabebordbar, M., Gorbatov, R., Sena, B., Nahrendorf, M., Briscoe, D. M., Li, R. A., Wagers, A. J., Rossi, D. J., Pu, W. T., and Chien, K. R. (2013) Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardialinfarction, Nat. Biotechnol., 31, 898–907.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., and Yamanaka, S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors, Cell, 131, 861–872.
Yakubov, E., Rechavi, G., Rozenblatt, S., and Givol, D. (2010) Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors, Biochem. Biophys. Res. Commun., 394, 189–193.
Lee, J., Sayed, N., Hunter, A., Au, K. F., Wong, W. H., Mocarski, E. S., Pera, R. R., Yakubov, E., and Cooke, J. P. (2012) Activation of innate immunity is required for efficient nuclear reprogramming, Cell, 151, 547–558.
Doyon, Y., McCammon, J. M., Miller, J. C., Faraji, F., Ngo, C., Katibah, G. E., Amora, R., Hocking T. D., Zhang, L., Rebar, E. J., Gregory, P. D., Urnov, F. D., and Amacher, S. L. (2008) Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases, Nat. Biotechnol., 26, 702–708.
Tesson, L., Usal, C., Menoret, S., Leung, E., Niles, B. J., Remy, S., Santiago, Y., Vincent, A. I., Meng, X., Zhang, L., Gregory, P. D., Anegon, I., and Cost, G. J. (2011) Knockout rats generated by embryo microinjection of TALENs, Nat. Biotechnol., 29, 695–696.
Wang, H., Yang, H., Shivalila, C. S., Dawlaty, M. M., Cheng, A. W., Zhang, F., and Jaenisch, R. (2013) Onestep generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering, Cell, 153, 910–918.
Wilber, A., Frandsen, J. L., Geurts, J. L., Largaespada, D. A., Hackett, P. B., and McIvor, R. S. (2006) RNA as a source of transposase for sleeping beauty-mediated gene insertion and expression in somatic cells and tissues, Mol. Ther., 13, 625–630.
Furushima, K., Jang, C. W., Chen, D. W., Xiao, N., Overbeek, P. A., and Behringer, R. R. (2012) Insertional mutagenesis by a hybrid piggyBac and sleeping beauty transposon in the rat, Genetics, 192, 1235–1248.
Bire, S., Gosset, D., Jegot, G., Midoux, P., Pichon, C., and Rouleux-Bonnin, F. (2013) Exogenous mRNA delivery and bioavailability in gene transfer mediated by piggyBac transposition, BMC Biotechnol., 13, 75.
Warren, L., Manos, P. D., Ahfeldt, T., Loh, Y. H., Li, H., Lau, F., Ebina, W., Mandal, P. K., Smith, Z. D., Meissner, A., Daley, G. Q., Brack, A. S., Collins, J. J., Cowan, C., Schlaeger, T. M., and Rossi, D. J. (2010) Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA, Cell Stem Cell, 7, 618–630.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O. V. Sergeeva, V. E. Koteliansky, T. S. Zatsepin, 2016, published in Biokhimiya, 2016, Vol. 81, No. 7, pp. 937-952.
To whom correspondence should be addressed.
Rights and permissions
About this article
Cite this article
Sergeeva, O.V., Koteliansky, V.E. & Zatsepin, T.S. mRNA-based therapeutics–Advances and perspectives. Biochemistry Moscow 81, 709–722 (2016). https://doi.org/10.1134/S0006297916070075
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297916070075