Abstract
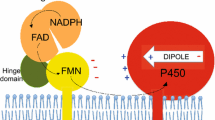
To understand the role of the structural elements of cytochrome b 5 in its interaction with cytochrome P450 and the catalysis performed by this heme protein, we carried out comparative structural and functional analysis of the two major mammalian forms of membrane-bound cytochrome b 5 — microsomal and mitochondrial, designed chimeric forms of the heme proteins in which the hydrophilic domain of one heme protein is replaced by the hydrophilic domain of another one, and investigated the effect of the highly purified native and chimeric heme proteins on the enzymatic activity of recombinant cytochromes P4503A4 and P45017A1 (CYP3A4 and CYP17A1). We show that the presence of a hydrophobic domain in the structure of cytochrome b 5 is necessary for its effective interaction with its redox partners, while the nature of the hydrophobic domain has no significant effect on the ability of cytochrome b 5 to stimulate the activity of cytochrome P450-catalyzed reactions. Thus, the functional properties of cytochrome b 5 are mainly determined by the structure of the hemebinding domain.
Similar content being viewed by others
References
Giordano, S. J., and Steggles, A. W. (1993) Biochim. Biophys. Acta, 1172, 95–100.
Altuve, A., Silchenko, S., Lee, K. H., Kuczera, K., Terzyan, S., Zhang, X., Benson, D. R., and Rivera, M. (2001) Biochemistry, 40, 9469–9483.
Kuroda, R., Ikenoue, T., Honsho, M., Tsujimoto, S., Mitoma, J. Y., and Ito, A. (1998) J. Biol. Chem., 273, 31097–31102.
Altuve, A., Wang, L., Benson, D. R., and Rivera, M. (2004) Biochem. Biophys. Res. Commun., 314, 602–609.
Enoch, H. G., Fleming, P. J., and Strittmatter, P. (1979) J. Biol. Chem., 254, 6483–6488.
Keyes, S. R., Alfano, J. A., Jansson, I., and Cinti, D. L. (1979) J. Biol. Chem., 254, 7778–7784.
Paltuaf, F., Prough, R. A., Masters, B. S., and Johnston, J. M. (1974) J. Biol. Chem., 249, 2661–2662.
Fukushima, H., Grinstead, G. F., and Gaylor, J. L. (1981) J. Biol. Chem., 256, 4822–4826.
Gacon, G., Lostanlen, D., Labie, D., and Kaplan, J. C. (1980) Proc. Natl. Acad. Sci. USA, 77, 1917–1921.
Livingston, D. J., McLachlan, S. J., La Mar, G. N., and Brown, W. D. (1985) J. Biol. Chem., 260, 15699–15707.
Strittmatter, P., Spatz, L., Corcoran, D., Rogers, M. J., Setlow, B., and Redline, R. (1974) Proc. Natl. Acad. Sci. USA, 71, 4565–4569.
Guryev, O. L., Gilep, A. A., Usanov, S. A., and Estabrook, R. W. (2001) Biochemistry, 40, 5018–5031.
Honsho, M., Mitoma, J. Y., and Ito, A. (1998) J. Biol. Chem., 273, 20860–20866.
Borgese, N., Gazzoni, I., Barberi, M., Colombo, S., and Pedrazzini, E. (2001) Mol. Biol. Cell, 12, 2482–2496.
Ito, A. (1980) J. Biochem., 87, 73–80.
Enoch, H. G., and Strittmatter, P. (1979) J. Biol. Chem., 254, 8976–8981.
Gilep, A. A., Guryev, O. L., Shet, M., Fisher, C., Usanov, S. A., and Estabrook, R. (1999) Mol. Steroidogenesis, 29, 85–86.
Chudaev, M. V., Gilep, A. A., and Usanov, S. A. (2001) Biochemistry (Moscow), 66, 667–681.
Sergeev, G. V., Gilep, A. A., Estabrook, R. W., and Usanov, S. A. (2006) Biochemistry (Moscow), 71, 790–799.
Sergeev, G. V., Gilep, A. A., Usanov, S. A., and Vasilevskaya, A. V. (2010) Trudy BGU. Mol. Biol., 5, 179–184.
Shen, A. L., Porter, T. D., Wilson, T. E., and Kasper, C. B. (1989) J. Biol. Chem., 264, 7584–7589.
Laemmli, U. K. (1970) Nature, 227, 680–685.
Chudaev, M. V., and Usanov, S. A. (1997) Biochemistry (Moscow), 62, 401–411.
Harlow, G. R., and Halpert, J. R. (1997) J. Biol. Chem., 272, 5396–5402.
Chung, B. C., Picado-Leonard, J., Haniu, M., Bienkowski, M., Hall, P. F., Shively, J. E., and Miller, W. L. (1987) Proc. Natl. Acad. Sci. USA, 84, 407–411.
Grigoryev, D. N., Kato, K., Njar, V. C., Long, B. J., Ling, Y. Z., Wang, X., Mohler, J., and Brodie, A. M. (1999) Anal. Biochem., 267, 319–330.
Soucy, P., and Luu-The, V. (2002) J. Steroid Biochem. Mol. Biol., 80, 71–75.
Perret, A., and Pompon, D. (1998) Biochemistry, 37, 11412–11424.
Schenkman, J. B., Voznesensky, A. I., and Jansson, I. (1994) Arch. Biochem. Biophys., 314, 234–241.
Yamazaki, H., Johnson, W. W., Ueng, Y. F., Shimada, T., and Guengerich, F. P. (1996) J. Biol. Chem., 271, 27438–27444.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2014, Vol. 79, No. 5, pp. 520–531.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM13-318, March 30, 2014.
Rights and permissions
About this article
Cite this article
Sergeev, G.V., Gilep, A.A. & Usanov, S.A. The role of cytochrome b 5 structural domains in interaction with cytochromes P450. Biochemistry Moscow 79, 406–416 (2014). https://doi.org/10.1134/S0006297914050046
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297914050046