Abstract
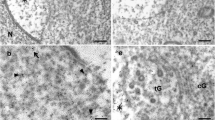
Acrosome is a specialized organelle in spermatozoids necessary for fertilization of oocyte. Acrosome is formed, according to various theories, either from vesicles of the Golgi apparatus or from endosomes and lysosomes. Hepatocyte growth factor regulated tyrosine kinase substrate (Hrs) is a multidomain protein component of ESCRT-0 complex, that participates in sorting of proteins absorbed during endocytosis. It has been shown that, in mammals, Hgs protein (Hrs homologue) possesses the ability to bind the acrosome, but whether Drosophila Hrs has a similar capacity has not been determined. We found that two forms of Hrs protein are expressed in Drosophila testes: a long form, Hrs-B, and a truncated form, Hrs-A. The later lacks a VHS domain and a portion of FYVE domain, both directly involved in Hrs anchoring to endosomes. We also determined that, unlike mammal Hgs, Drosophila Hrs-B isoform and an almost identical truncated form, Hrs8 ([290–760]), are localized not to acrosome, but to spermatocyte fusomes. This localization requires a portion of the protein molecule located between amino acid residues 383 and 472, which presumably correspond to NF2- and/or Stam-binding domains. In situ hybridization for hrs mRNA showed that the gene is expressed during early spermatogenesis. This is consistent with our data that the Hrs protein binds to spermatocyte fusomes and is absent during later spermatogenesis stages. In addition, we have shown that Hrs is not involved in regulation or implementation of cytokinesis in spermatocytes. Finally, despite the absence of Hrs on Drosophila acrosomes, we detected endosome markers Rab4, Rab7, and Rab11 on this organelle. Thus, our data support the endosomal hypothesis of acrosome biogenesis.
Similar content being viewed by others
Abbreviations
- MVB:
-
multivesicular bodies
- ESCRT:
-
endosomal sorting complex required for transport
- STAM:
-
signal-transducing adaptor molecule
References
Asao, H., Sasaki, Y., Arita, T., Tanaka, N., Endo, K., Kasai, H., Takeshita, T., Endo, Y., Fujita, T., and Sugamura, K., HRS is associated with STAM, a signal-transducing adaptor molecule. Its suppressive effect on cytokine induced cell growth, J. Biol. Chem., 1997, vol. 272, pp. 32785–32791.
Berruti, G., Ripolone, M., and Ceriani, M., USP8, a regulator of endosomal sorting, is involved in mouse acrosome biogenesis: through interaction with the spermatid ESCRT-0 complex and microtubules, Biol. Reproduct., 2010, vol. 82, pp. 930–939.
Burgos, M.H. and Gutierrez, L.S., The Golgi complex of the early spermatid in guinea pig, Anat. Rec., 1986, vol. 216, pp. 139–145.
Capalbo, L., Montembault, E., Takeda, T., Bassi, Z.I., Glover, D.M., and D’Avino, P.P., The chromosomal passenger complex controls the function of endosomal sorting complex required for transport-III Snf7 proteins during cytokinesis, Open Biol., 2012, vol. 2, p. 120070.
Carlton, J.G. and Martin-Serrano, J., Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery, Science, 2007, vol. 316, pp. 1908–1912.
Chen, D. and McKearin, D.M., A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell, Development, 2003, vol. 130, pp. 1159–7110.
Dorogova, N.V., Akhmametyeva, E.M., Kopyl, S.A., Gubanova, N.V., Yudin, O.S., Omelyanchuk, L.V., and Chang, L.S., The role of Drosophila Merlin in spermatogenesis, BMC Cell Biol., 2008, vol. 9, p. 1.
Fuller, M., Spermatogenesis, in The Development of Drosophila melanogaster, New York: Cold Spring Harbor Laboratory Press, 1993, pp. 71–147.
Giansanti, M.G., Belloni, G., and Gatti, M., Rab11 is required for membrane trafficking and actomyosin ring constriction in meiotic cytokinesis of Drosophila males, Mol. Biol. Cell, 2007, vol. 18, pp. 5034–5047.
Hariharan, I.K. and Bilder, D., Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila, Annu. Rev. Genet., 2006, vol. 40, pp. 335–361.
Hayakawa, A. and Kitamura, N., Early endosomal localization of hrs requires a sequence within the prolineand glutamine-rich region but not the FYVE finger, J. Biol. Chem., 2000, vol. 275, pp. 29636–29642.
Hofmann, K. and Falquet, L., A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems, Trends Biochem. Sci., 2001, vol. 26, pp. 347–350.
Li, S., Qiao, Y., Di, Q., Le, X., Zhang, L., Zhang, X., Zhang, C., Cheng, J., Zong, S., Koide, S.S., Miao, S., and Wang, L., Interaction of SH3P13 and DYDC1 protein: a germ cell component that regulates acrosome biogenesis during spermiogenesis, Eur. J. Cell Biol., 2009, vol. 88, pp. 509–520.
Li, Y.C., Hu, X.Q., Zhang, K.Y., Guo, J., Hu, Z.Y., Tao, S.X., Xiao, L.J., Wang, Q.Z., Han, C.S., and Liu, X.L., Afaf, a novel vesicle membrane protein, is related to acrosome formation in murine testis, FEBS Lett., 2006, vol. 5807, pp. 4266–4273.
Lohi, O. and Lehto, V.P., VHS domain marks a group of proteins involved in endocytosis and vesicular trafficking, FEBS Lett., 1998, vol. 440, pp. 255–257.
Lohi, O., Poussu, A., Mao, Y., Quiocho, F., and Lehto, V.P., VHS domain—a longshoreman of vesicle lines, FEBS Lett., 2002, vol. 513, pp. 19–23.
Martinez, M.J., Geuze, H.J., and Ballesta, J., Evidence for a nonlysosomal origin of the acrosome, J. Histochem. Cytochem., 1996, vol. 44, pp. 313–320.
Moreno, R.D. and Alvarado, C.P., The mammalian acrosome as a secretory lysosome: new and old evidence, Mol. Reprod. Dev., 2006, vol. 73, pp. 1430–1434.
Moreno, R.D., Palomino, J., and Schatten, G., Assembly of spermatid acrosome depends on microtubule organization during mammalian spermiogenesis, Dev. Biol., 2006, vol. 293, pp. 218–227.
Moreno, R.D., Ramalho-Santos, J., Chan, E.K., Wessel, G.M., and Schatten, G., The Golgi apparatus segregates from the lysosomal/acrosomal vesicle during rhesus spermiogenesis: structural alterations, Dev. Biol., 2000, vol. 219, pp. 334–349.
Morita, E., Sandrin, V., Chung, H.-Y., Morham, S.G., Gyg, S.P., Rodesch, C.K., and Sundquist, W.I., Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis, EMBO J., 2007, vol. 26, pp. 4215–4227.
Nagaso, H., Murat, T., Da, N., and Yokoyam, K., Simultaneous detection of RNA and protein A by in situ hybridization and immunological staining, J. Histochem. Cytochem., 2001, vol. 49, p. 1177.
Omelyanchuk, L.V., Pertseva, J.A., Burns, S.S., and Chang, L.S., Evolution and origin of HRS, a protein interacting with Merlin, the neurofibromatosis 2 gene product, Gene Regul. Syst. Biol., 2009, vol. 3, p. 143157.
Raiborg, C., Bache, K.G., Mehlum, A., and Stenmark, H., Function of HRS in endocytic trafficking and signaling, Biochem. Soc. Transact., 2001, vol. 29, pp. 473–475.
Stenmark, H. and Aasland, R., FYVE-finger proteinseffectors of an inositol lipid, J. Cell Sci., 1999, vol. 112, pp. 4175–4183.
Sun-Wada, G.H., Imai-Senga, Y., Yamamoto, A., Murata, Y., Hirata, T., Wada, Y., and Futai, M., A proton pump ATPase with testis-specific E1-subunit isoform required for acrosome acidification, J. Biol. Chem., 2002, vol. 277, pp. 18098–18105.
Takata, H., Kato, M., Denda, K., and Kitamura, N., A hrs binding protein having a Src homology 3 domain is involved in intracellular degradation of growth factors and their receptors, Genes Cells, 2000, vol. 5, pp. 57–69.
Takeshita, T., Arita, T., Higuchi, M., Asao, H., Endo, K., Kuroda, H., Tanaka, N., Murata, K., Ishii, N., and Sugamura, K., STAM, signal transducing adaptor molecule, is associated with Janus kinases and involved in signaling for cell growth and c-myc induction, Immunity, 1997, vol. 6, pp. 449–457.
Tang, X.M., Lalli, M.F., and Clermont, Y., A cytochemical study of the Golgi apparatus of the spermatid during spermiogenesis in the rat, Am. J. Anat., 1982, vol. 163, pp. 283–294.
Tognon, E., Wollscheid, N., Cortese, K., Tacchetti, C., and Vaccari, T., ESCRT-0 is not required for ectopic Notch activation and tumor suppression in Drosophila, PLoS One, 2014, vol. 9, p. e93987.
Wilson, K.L., Fitch, K.R., Bafus, B.T., and Wakimoto, B.T., Sperm plasma membrane breakdown during Drosophila fertilization requires Sneaky, an acrosomal membrane protein, Development, 2006, vol. 133, pp. 4871–4879.
Young, P., Deveraux, Q., Beal, R.E., Pickart, C.M., and Rechsteiner, M., Characterization of two polyubiquitin binding sites in the 26 S protease subunit 5a, J. Biol. Chem., 1998, vol. 273, pp. 5461–5467.
Zhang, J., Schulze, K.L., Hiesinger, P.R., Suyama, K., Wang, S., Fish, M., Acar, M., Hoskins, R.A., Bellen, H.J., and Scott, M.P., Thirty-one flavors of Drosophila Rab proteins, Genetics, 2007, vol. 176, pp. 1307–1322.
Zhu, G., Salazar, G., Zlatic, S.A., Fiza, B., Doucette, M.M., Heilman, C.J., Levey, A.J., Faundez, V., and L’Hernault, S.W., SPE-39 family proteins interact with the HOPS complex and function in lysosomal delivery, Mol. Biol. Cell., 2009, vol. 20, pp. 1223–1240.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Marilovtseva, T.D. Dubatolova, Ya. Galimova, S.A. Kopyl, L.V. Omelyanchuk, 2015, published in Tsitologiya, 2015, Vol. 57, No. 7, pp. 509–517.
Rights and permissions
About this article
Cite this article
Marilovtseva, E.V., Dubatolova, T.D., Galimova, Y.A. et al. A study of cellular localization of HRS and other endosome markers during spermatogenesis in Drosophila melanogaster using chimeric GFP constructs. Cell Tiss. Biol. 9, 452–461 (2015). https://doi.org/10.1134/S1990519X15060073
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X15060073