Abstract
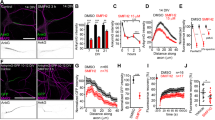
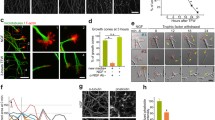
Although selective pruning of subsets of branches is a key step for axonal remodeling, the underlying mechanisms have not fully elucidated. We have reported that the retraction rate of axonal arbor is higher in the terminals, where less kinesin is sorted, and inhibiting actin turnover by treatment with latrunculin A prevented retraction in such terminals. In the present study, we investigated the effects of pharmacological inhibitors for the regulators of actin polymerization/destabilization on the elongation and retraction of arbor terminals in cerebellar granule neurons. Inhibition of an actin nucleator Arp2/3 with CK-666 or CK-869 inhibited the retraction of arbor terminals. However, inhibitors for PAK/LIMK, upstream signaling of ADF/cofilin, reduced the elongation rate but not the retraction rate. A photoconversion analysis revealed that turnover of F-actin at the tip of the axonal terminal is faster in the presence of the Arp2/3 inhibitor compared with the control and neurons treated with the LIMK inhibitor. Our results indicate the important role of Arp2/3 for the retraction of axonal arbor terminals in cerebellar granule neurons.


Similar content being viewed by others
REFERENCES
Pollard, T.D., Blanchoin, L., and Mullins, R.D., J. Cell Sci., 2001, vol. 114, pp. 3–4.
Pak, C.W., Flynn, K.C., and Bamburg, J.R., Nat. Rev. Neurosci., 2008, vol. 9, pp. 136–147.
Goley, E.D. and Welch, M.D., Nat. Rev. Mol. Cell. Biol., 2006, vol. 7, pp. 713–726.
Takenawa, T. and Miki, H., J. Cell Sci., 2001, vol. 114, pp. 1801–1809.
Korobova, F. and Svitkina, T., Mol. Biol. Cell., 2008, vol. 19, pp. 1561–1574.
San Miguel-Ruiz J.E. and Letourneau, P.C., J. Neurosci., 2014, vol. 34, pp. 5895–5908.
Strasser, G.A., Rahim, N.A., Van der Waal K.E., Gertler, F.B., and Lanier, L.M., Neuron, 2004, vol. 43, pp. 81–94.
Pinyol, R., Haeckel, A., Ritter, A., Qualmann, B., and Kessels, M.M., PLoS One, 2007, vol. 2, e400.
Oser, M. and Condeelis, J., J. Cell. Biochem., 2009, vol. 108, pp. 1252–1262.
Bravo-Cordero, J.J., Magalhaes, M.A., Eddy, R.J., Hodgson, L., and Condeelis, J., Nat. Rev. Mol. Cell. Biol., 2013, vol. 14, pp. 405–415.
Kovaleva, T.F., Maksimova, N.S., Zhukov, I.Y., Per-shin, V.I., Mukhina, I.V., and Gainullin, M.R., Neurochem. J., 2019, vol. 13, pp. 11–19.
Yang, N., Higuchi, O., Ohashi, K., Nagata, K., Wada, A., Kangawa, K., Nishida, E., and Mizuno, K., Nature, 1998, vol. 393, pp. 809–812.
Aizawa, H., Wakatsuki, S., Ishii, A., Moriyama, K., Sasaki, Y., Ohashi, K., Sekine-Aizawa, Y., Sehara-Fujisawa, A., Mizuno, K., Goshima, Y., and Yahara, I., Nat. Neurosci., 2001, vol. 4, pp. 367–373.
Ng, J. and Luo, L., Neuron, 2004, vol. 44, pp. 779–793.
Endo, M., Ohashi, K., Sasaki, Y., Goshima,Y., Niwa, R., Uemura, T., and Mizuno, K., J. Neurosci., 2003, vol. 23, pp. 2527–2537.
Dong, Q., Ji, Y.S., Cai, C., and Chen, Z.Y., J. Biol. Chem., 2012, vol. 287, pp. 41720–41731.
Luo, L. and O’Leary, D.D., Annu. Rev. Neurosci., 2005, vol. 28, pp. 127–156.
Gallo, G., Yee, H.F., and Letourneau, P.C., Jr., J. Cell Biol., 2002, vol. 158, pp. 1219–1228.
Gallo, G., J. Cell Sci., 2006, vol. 119, pp. 3413–3423.
Ruthel, G. and Hollenbeck, P.J., J. Neurosci., 2000, vol. 20, pp. 2266–2274.
Hutchins, B.I. and Kalil, K., J. Neurosci., 2008, vol. 28, pp. 143–153.
Nakata, T. and Hirokawa, N., J. Cell Biol., 2003, vol. 162, pp. 1045–1055.
Jacobson, C., Schnapp, B., and Banker, G.A., Neuron, 2006, vol. 49, pp. 797–804.
Seno, T., Ikeno, T., Mennya, K., Kurishita, M., Sakae, N., Sato, M., Takada, H., and Konishi, Y., J. Cell Sci. 2016, vol. 129, pp. 3499–3510.
Konishi, Y., Stegmüller, J., Matsuda, T., Bonni, S., and Bonni, A., Science, 2004, vol. 303, pp. 1026–1030.
Kubota, K., Seno, T., and Konishi, Y., Brain Res., 2013, vol. 1539, pp. 15–23.
Konishi, Y. and Setou, M., Nat. Neurosci., 2009, vol. 12, pp. 559–567.
Mckinney, S.A., Murphy, C.S., Hazelwood, K.L., Davidson, M.W., and Looger, L.L., Nat. Methods, 2009, vol. 6, pp. 131–133.
Inami, Y., Omura, M., Kubota, K., and Konishi, Y., Brain Res., 2018, vol. 1690, pp. 51–60.
Kanchanawong, P., Shtengel, G., Pasapera, A.M., Ramko, E.B., Davidson, M.W., Hess H.F., and Waterman C.M., Nature, 2010, vol. 468, pp. 580–584.
Hetrick, B., Han, M.S., Helgeson, L.A., and Nolen, B.J., Chem. Biol., 2013, vol. 20, pp. 701–712.
ACKNOWLEDGMENTS
We thank Dr. Michael Davidson for providing the constructs used in this study.
Funding
This study was funded by grants from the JSPS KAKENHI Grant (no. 17K07107).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare no conflicts of interest associated with this manuscript.
Ethical approval. All animals were treated according to the ethical guidelines from the Central Institute for Experimental Animals (CIEA, Japan). The experimental protocols were approved by the animal experiment committee of the University of Fukui.
Additional information
Abbreviations: Arp, actin-related protein; PAK, p21-activated kinase; LIMK, LIM kinase; ADF, actin-depolymerizing factor; CGN, cerebellar granule neuron; DMSO; dimethyl sulfoxide; MEM, minimal essential medium.
Rights and permissions
About this article
Cite this article
Ikeno, T., Konishi, Y. Arp2/3 Is Required for Axonal Arbor Terminal Retraction in Cerebellar Granule Neurons. Neurochem. J. 14, 32–36 (2020). https://doi.org/10.1134/S1819712420010109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1819712420010109