Abstract
The aim of this work was to design and characterize peptides based on the α-helices h1 and h2 of the ACE2 receptor, forming the interaction interface between the receptor-binding domain (RBD) of the SARS-CoV-2 S protein and the cellular ACE2 receptor. Monomeric and heterodimeric peptides connected by disulfide bonds at different positions were synthesized. Solubility, RBD-binding affinity, and peptide helicity were experimentally measured, and molecular dynamics simulation was performed in various solvents. It was established that the preservation of the helical conformation is a necessary condition for the binding of peptides to RBD. The peptides have a low degree of helicity and low affinity for RBD in water. Dimeric peptides have a higher degree of helicity than monomeric ones, probably due to the mutual influence of helices. The degree of helicity of the peptides in trifluoroethanol is the highest; however, for in vitro studies, the most suitable solvent is a water-ethanol mixture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The COVID-19 pandemic and the emergence of new variants of its etiological agent, the SARS-CoV-2 virus, make studies on the search for effective tools for prevention and treatment of this infection relevant. At the initial stages of the interaction of the virus with the cell, the key role is played by the S protein, which forms spikes on the surface of the viral particle. The receptor-binding domain (RBD) of the S protein interacts with the interface of the cellular ACE2 receptor, which consists of h1 and h2 α-helices, and triggers the fusion of the cell and viral membranes for the penetration of the viral genome into the cytoplasm [1]. To date, to inhibit this interaction, monoclonal antibodies with neutralizing activity have been developed and used in clinical practice [2–4], which will later become a reference drug for new compounds. One of the approaches to design of the molecules capable of competitively preventing the binding of RBD of SARS-CoV-2 and related viruses to ACE2 is the design of peptides that mimic the structure and properties of the cellular ACE2 receptor. In 2020–2022, a significant number of peptide molecules based on the ACE2 sequence and aimed at interacting with the S protein [5] have been studied in silico, and only for some of them the effectiveness of inhibition of the formation of the RBD-ACE2 complex and (or) antiviral activity have been studied in vitro. Moreover, a few of synthesized compounds that exhibited a high (nanomolar) binding affinity in silico [6] and in vitro [7, 8] exhibited antiviral activity in cell or animal models [9–12].
To increase the binding efficiency, it is necessary to optimize the peptide structure in order to create a molecule that most closely resembles the ACE2 structure and provides a high-affinity interaction with RBD [13–15]. We have developed and synthesized monomeric peptides based on the amino acid sequence of antiparallel h1 and h2 ACE2 α-helices with modified residues. Monomeric peptides were linked by disulfide bridges at different positions to obtain heterodimeric “chimeric” peptides X1–4 (Table 1; synthesis of peptides X1, X2, and their monomers was described earlier [16]). For these peptides, experimental studies of solubility, binding affinity with RBD, and the degree of helicity, as well as molecular dynamics simulations in various solvents, were performed.
Affinity was studied by microthermophoresis [17], and the degree of α-helicity was studied by circular dichroism (CD) spectroscopy [18] (Table 1). The synthesized peptides are characterized by low water solubility. In aqueous solution, only two chimeric peptides (Kd X1: 4 μM, X2: 12 μM) and two monomeric peptides based on the h2 helix showed affinity for RBD (dissociation constants Kd were 40 μM for h2 and 2 μM for 900h2). It was found by the CD method that the peptides in aqueous solution have a low degree of helicity, which may be the cause for their low affinity for RBD.
Stabilization of secondary structure elements in peptides and proteins (in particular, α-helices) is a complex multifactorial process involving several different mechanisms. The spiralization-inducing effects include a decrease in the entropy contribution of solvent molecules due to the distortion of the hydrogen bond network between them and the side chains of amino acid residues in the peptide.
To increase the degree of helicity of the synthesized peptides, we selected solvents that stabilize their α-helical conformation and are also suitable for biological experiments.
The most suitable solvent for maintaining a stable helical conformation of peptides is trifluoroe-thanol (TFE) [19]. The degree of peptide helicity in it was the highest (from 50 to 100%). The dimeric peptides X3 and X4 retained a completely helical conformation in TFE. This solvent can be used to characterize the overall spiralization ability of the peptide. However, TFE is toxic to cell cultures and is unsuitable for in vitro studies at high concentrations.
The CD spectroscopy confirmed a higher degree of helicity of peptides in a water–ethanol mixture (WEM) (37–63%) compared to water. In a water–alcohol mixture, monomeric peptides exhibit affinity for RBD with dissociation constants in the range of 1‒4 and 2–8 μM for peptides based on the h1 and h2 α-helix sequences, respectively. Combining monomeric peptides into heterodimers leads to an increase in affinity when connecting “middle to middle” or “tail to head” (dissociation constants of dipeptides X2 and X4 < 1 μM) but does not influence affinity when connecting “head to tail” (dipeptide dissociation constant X1 ~7 μM) (Table 1). In addition, dimeric peptides have a higher degree of helicity than monomeric ones, which may be due to the mutual stabilizing effect of h1 and h2 helices.
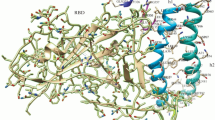
In a molecular dynamics simulation (100–300 ns) in water, peptides, especially monomeric ones, tend to destabilize the structure and lose their helical conformation (Fig. 1).
Representative structures of the monomeric peptides 200h1 (a) and 900h2 (b) and the chimeric peptide X3 derived from them (c) according to the results of molecular dynamics simulations. Structures in water, WEM, and TFE are colored in blue, red, and yellow, respectively, and superimposed by structural similarity. The superposition of the 200h1 structures is impossible due to the fundamental difference in the conformations.
The degree of helicity and stability of chimeric peptides in water is significantly higher than that of monomeric peptides, therefore supporting the hypothesis of mutual stabilization of helices in heterodimers. In a water–ethanol mixture and in TFE, the helical conformation remains fairly stable during the entire simulation time for both monomeric and chimeric peptides. This indicates the possibility of additional stabilization of the helical structure due to the properties of these solvents, which partially mimic the features of the protein environment of the helices in the receptor structure.
The proposed dimeric peptides based on the h1 and h2 helices of the ACE2 receptor connected by disulfide bridges, are capable of binding to the RBD of the SARS-CoV-2 S protein with an affinity in the submicromolar range, which exceeds the affinity of individual monomers. The formation of a helical structure of peptides increases the efficiency of binding to SARS-CoV-2 RBD: the affinity of peptides to RBD depends on the degree of α-helicity of their secondary structure, and the preservation of the original helical structure inherent in the ACE2 receptor is necessary for successful binding of the SARS-CoV-2 S-protein to RBD. The water–ethanol mixture maintains the helicity of the peptides and can be used for in vitro studies. Further stabilization of the peptide structure and selection of the optimal amino acid sequence and solvent should make it possible to design candidate molecules for studying the interaction between SARS-CoV-2 and the receptor in vivo.
REFERENCES
Yang, J., Petitjean, S.J.L., Koehler, M., et al., Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor, Nat. Commun., 2020, vol. 11, p. 4541.
Guo, Y., Huang, L., Zhang, G., et al., SARS-CoV-2 neutralizing antibody with extensive spike binding coverage and modified for optimal therapeutic outcomes, Nat. Commun., 2021, vol. 12, no. 1, p. 2623.
Zhou, X., Wang, H., Ji, Q., et al., Molecular deconvolution of the neutralizing antibodies induced by an inactivated SARS-CoV-2 virus vaccine, Protein Cell, 2021, vol. 12, no. 10, pp. 818–823.
Chen, D., Zhao, Y., Li, M., et al., A general Fc engineering platform for the next generation of antibody therapeutics, Theranostics, 2021, vol. 11, no. 4, pp. 1901–1917.
Pomplun, S., Targeting the SARS-CoV-2-spike protein: from antibodies to miniproteins and peptides, RSC Med. Chem., 2020, vol. 12, no. 2, pp. 197–202.
Han, Y. and Král, P., Computational design of ACE2-based peptide inhibitors of SARS-CoV-2, ACS Nano, 2020, vol. 14, no. 4, pp. 5143–5147.
Huang, X., Pearce, R., and Zhang, Y., De novo design of protein peptides to block association of the SARS-CoV-2 spike protein with human ACE2, Aging (Albany, NY), 2020, vol. 12, no. 12, pp. 11263–11276.
Sadremomtaz, A., Al-Dahmani, Z.M., Ruiz-Moreno, A.J., et al., Synthetic peptides that antagonize the angiotensin-converting enzyme-2 (ACE-2) interaction with SARS-CoV-2 receptor binding spike protein, J. Med. Chem., 2022, vol. 65, no. 4, pp. 2836–2847.
Cao, L., Goreshnik, I. Coventry, B., et al., De novo design of picomolar SARS-CoV-2 miniprotein inhibitors, Science, 2020, vol. 370, no. 6515, pp. 426–431.
Curreli, F., Victor, S.M.B., Ahmed, S., et al., Stapled peptides based on human angiotensin-converting enzyme 2 (ACE2) potently inhibit SARS-CoV-2 infection in vitro, mBio, 2020, vol. 11, no. 6, p. e02451–20.
Khatri, B., Pramanick, I., Malladi, S.K., et al., A dimeric proteomimetic prevents SARS-CoV-2 infection by dimerizing the spike protein, Nat. Chem. Biol., 2022.
Linsky, T.W., Vergara, R., Codina, N., et al., De novo design of potent and resilient hACE2 decoys to neutralize SARS-CoV-2, Science, 2020, vol. 370, no. 6521, pp. 1208–1214.
Muttenthaler, M., King, G.F., Adams, D.J., and Alewood, P.F., Trends in peptide drug discovery, Nat. Rev. Drug. Discov., 2021, vol. 20, no. 4, pp. 309–325.
Maas, M.N., Hintzen, J.C.J., Loffler, P.M.G., et al., Targeting SARS-CoV-2 spike protein by stapled hACE2 peptides, Chem. Commun. (Camb.), 2021, vol. 7, no. 26, pp. 3283–3286.
Morgan, D.C., Morris, C., Mahindra, A., et al., Stapled ACE2 peptidomimetics designed to target the SARS-CoV-2 spike protein do not prevent virus internalization, Pept. Sci. (Hoboken), 2021, vol. 8, p. e24217.
Bibilashvili, R.Sh., Sidorova, M.V., Dudkina, U.S., et al., Peptide inhibitors of the interaction of the receptor-binding domain of the SARS-CoV-2 virus with the cellular ACE2 receptor, Biomed. Khim., 2021, vol. 67, no. 3, pp. 244–250.
Tsvetkov, V., Varizhuk, A., Kozlovskaya, L., et al., EGCG as an anti-SARS-CoV-2 agent: preventive versus therapeutic potential against original and mutant virus, Biochimie, 2021, no. 191, pp. 27–32.
Sreerama, N.W., Estimation of protein secondary structure from circular dichroism spectra: comparison of contin, selcon, and cdsstr methods with an expanded reference set, Anal. Biochem., 2020, vol. 287, no. 2, pp. 252–260.
Buck, M., Trifluoroethanol and colleagues: cosolvents come of age. Recent studies with peptides and proteins, Q. Rev. Biophys., 1998, vol. 31, no. 3, pp. 297–355.
ACKNOWLEDGMENTS
We are grateful to V. Manuvera and A. Varizhuk for assessing the binding affinity by thermophoresis.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 20-04-60110).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Batrukova
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sidorova, M.V., Bibilashvili, R.S., Avdeev, D.V. et al. Properties and Activity of Peptide Derivatives of ACE2 Cellular Receptor and Their Interaction with SARS-CoV-2 S Protein Receptor-Binding Domain. Dokl Biochem Biophys 507, 237–241 (2022). https://doi.org/10.1134/S1607672922060126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1607672922060126