Abstract
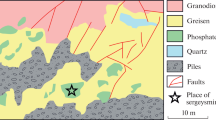
The paper describes the first finding of quintinite [Mg4Al2(OH)12][(CO3)(H2O)3] at the Mariinsky deposit in the Central Urals, Russia. The mineral occurs as white tabular crystals in cavities within altered gabbro in association with prehnite, calcite, and a chlorite-group mineral. Quintinite is the probable result of late hydrothermal alteration of primary mafic and ultramafic rocks hosting emerald-bearing glimmerite. According to electron microprobe data, the Mg: Al ratio is ~2: 1. IR spectroscopy has revealed hydroxyl and carbonate groups and H2O molecules in the mineral. According to single crystal XRD data, quintinite is monoclinic, space group C2/m, a =5.233(1), b = 9.051(2), c = 7.711(2) Å, β = 103.09(3)°, V = 355.7(2) Å3. Based on structure refinement, the polytype of quintinite should be denoted as 1M. This is the third approved occurrence of quintinite-1M in the world after the Kovdor complex and Bazhenovsky chrysotile–asbestos deposit.
Similar content being viewed by others
References
Arakcheeva, A.V., Pushcharovskii, D.Yu., Atencio, D., and Lubman, G.U., Crystal structure and comparative crystal chemistry of Al2Mg4(OH)12(CO3) · 3H2O, a new mineral from the hydrotalcite–manasseite group, Crystallogr. Rep., 1996, vol. 41, pp. 972–981.
Bonaccorsi, E., Merlino, S., and Orlandi, P., Zincalstibite, a new mineral, and cualstibite: crystal chemical and structural relationships, Am. Mineral., 2007, vol. 92, pp. 198–203.
Braithwaite, R.S.W., Dunn, P.J., Pritchard, R.G., and Paar, W.H., Iowaite, a re-investigation, Mineral. Mag., 1994, vol. 58, pp. 79–85.
Britvin, S.N. Structural diversity of layered double hydroxides, in Minerals as Advanced Materials, Krivovichev S. V., Ed., Berlin: Springer, 2008, pp. 123–128.
Chao, G.Y. and Gault, R.A., Quintinite-2H, quintinite-3T, charmarite-2H, charmarite-3T, and caresite-3T, a new group of carbonate minerals related to the hydrotalcite/ manasseite group, Can. Mineral., 1997, vol. 35, pp. 1541–1549.
Chukanov, N.S. and Chervonnyi, A.D., Infrared Spectroscopy of Minerals and Related Compounds, Springer Mineralogy, 2016.
Cooper, M.A. and Hawthorne, F.C., The crystal structure of shigaite, [AlMn2(OH)6]3(SO4)2Na(H2O)6{H2O}6, a hydrotalcite-group mineral, Can. Mineral., 1996, vol. 34, pp. 91–97.
Drits, V.A., Sokolova, T.N., Sokolova, G.V., and Cherkashin, V.I., New members of the hydrotalcite–manasseite group. Clays Clay Miner., 1987, vol. 35, no. 6, pp. 401–417.
Feoktistov, G.D., Ivanov, S.I., Kashaev, A.A., Klyuchisnkiy, L.N., Taskina, N.G., and Ushchapovskaya, Z.F., On the finding of chlor-manasseite in the USSR, Zap. Ross. Mineral. O-va, 1978, vol. 107, no. 3, pp. 321–325.
Huminicki, D.M.C. and Hawthorne, F.C., The crystal structure of nikischerite, NaFeAl3(SO4)2(OH)18(H2O)12, a mineral of the shigaite group, Can. Mineral., 2003, vol. 41, pp. 79–82.
Koch, C.B., Structures and properties of anionic clay minerals, Hyperfine Interact., 1998, vol. 117, no. 1, pp. 131–157.
Kolitsch, U., Giester, G., and Pippinger, T., The crystal structure of cualstibite-1M (formerly cyanophyllite), its revised chemical formula and its relation to cualstibite-1T, Mineral. Petrol., 2013, vol. 107, pp. 171–178.
Krivovichev, S.V., Yakovenchuk, V.N., Zhitova, E.S., Zolotarev, A.A., Pakhomovsky, Y.A., and Ivanyuk, G.Y., Crystal chemistry of natural layered double hydroxides. 1. Quintinite-2H-3c from the Kovdor alkaline massif, Kola Peninsula, Russia, Mineral. Mag., 2010a, vol. 74, no. 5, pp. 821–832.
Krivovichev, S.V., Yakovenchuk, V.N., Zhitova, E.S., Zolotarev, A.A., Pakhomovsky, Y.A., and Ivanyuk, G.Yu., Crystal chemistry of natural layered double hydroxides. 2. Quintinite-1M: first evidence of a monoclinic polytype in M2+–M3+ layered double hydroxides, Mineral. Mag., 2010b, vol. 74, no. 5, pp. 833–840.
Krivovichev, S.V., Antonov, A.A., Zhitova, E.S., Zolotarev, A.A., Krivovichev, V.G., and Yakovenchuk, V.N., Quintinite-1M from Bazhenovskoe deposit (Middle Urals, Russia): crystal structure and properties, Vestn. St. Peterburg. Gos. Univ., Ser. 7, Geol. Geogr., 2012, vol. 7, no. 2, pp. 3–9.
Layered Double Hydroxides. Structure and Bonding, Duan, X. and Evans, D.G., Eds., Berlin: Springer, 2006.
Lisitsina, V.A., Drits, V.A., Sokolova, G.V., and Aleksandrova, V.A., New complex of secondary minerals-products of low temperature transformations of rocks, covering basalts of Atlantic Ocean underwater mountains, Litol. Polezn. Iskop., 1985, vol. 6, pp. 20–39.
Mills, S.J., Christy, A.G., Genin, J.-M.R., Kameda, T., and Colombo, F., Nomenclature of the hydrotalcite supergroup: natural layered double hydroxides, Mineral. Mag., 2012a, vol. 76, pp. 1289–1336.
Mills, S.J., Christy, A.G., Kampf, A.R., Housley, R.M., Favreau, G., Boulliard, J.-C., and Bourgoin, V., Zincalstibite-9R: the first nine-layer polytype with the layered double hydroxide structure-type, Mineral. Mag., 2012b, vol. 76, pp. 1337–1345.
Mills, S.J., Kampf, A.R., Housley, R.M., Favreau, G., Pasero, M., Biagioni, C., Merlino, S., Berbain, C., and Orlandi, P., Omsite, (Ni,Cu)2Fe3+(OH)6[Sb(OH)6], a new member of the cualstibite group from Oms, France, Mineral. Mag., 2012c, vol. 76, pp. 1347–1354.
Mills, S.J., Whitfield, P.S., Kampf, A.R., Wilson, S.A., Dipple, G.M., Raudsepp, M., and Favreau, G., Contribution to the crystallography of hydrotalcites: the crystal structures of woodallite and takovite, J. Geosci., 2012d, vol. 58, pp. 273–279.
Rajamathi, M., Thomas, G.S., and Kamath, P.V., The many ways of making anionic clays, J. Chem. Sci, 2001, vol. 113, no. 5, pp. 671–680.
Reichle, W.T., Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite), Solid State Ionics, 1986, vol. 22, no. 1, pp. 135–141.
Rius, J. and Allmann, R., The superstructure of the double layer mineral wermlandite, [Mg7(Al0.57, Fe0.43 3+)(OH)0.43)2]2+[(Ca0.6,Mg0.4)(SO4)2(H2O)12]2–, locality: Langban, Warmland, Sweden, Z. Kristallogr., 1984, vol. 168, pp. 133–144.
Sacerdoti, M. and Passaglia, E., Hydrocalumite from Latium, Italy: its crystal structure and relationship with related synthetic phases, N. Jb. Miner. Monatsch., 1988, pp. 462–475.
Shannon, R.D., Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., 1970, vol. A32, pp. 751–767.
Sheldrick, G.M., A short history of SHELX, Acta Crystallogr., 2008, vol. A64, pp. 112–122.
Taylor, S.R., Abundance of chemical elements in the continental crust: a new table, Geochim. Cosmochim. Acta, 1964, vol. 28, pp. 1273–1285.
Theiss, F., Lopez, A., Frost, R.L., and Scholz, R., Spectroscopic characterisation of the LDH mineral quintinite Mg4Al2(OH)12CO3 · 3H2O, Spectrochim. Acta A, 2015, vol. 150, pp. 758–64.
Walenta, K., Cualstibite, a new secondary mineral from the Clara mine in the Central Black Forest (FRG), Chem. Erde, 1984, vol. 43, pp. 255–260.
Zhitova, E.S., Crystal chemistry of natural layered double hydroxides, Saint Petersburg State University Studies in Earth Sciences, St. Petersburg University Press, 2013, vol. 1.
Zhitova, E.S., Yakovenchuk, V.N., Krivovichev, S.V., Zolotarev, A.A., Pakhomovsky, Y.A., and Ivanyuk, G.Y., Crystal chemistry of natural layered double hydroxides. 3. The crystal structure of Mg, Al-disordered quintinite-2H, Mineral. Mag., 2010, vol. 74, no. 5, pp. 841–848.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.S. Zhitova, M.P. Popov, S.V. Krivovichev, A.N. Zaitsev, N.S. Vlasenko, 2016, published in Zapiski Rossiiskogo Mineralogicheskogo Obshchestva, 2016, No. 6, pp. 90–101.
Rights and permissions
About this article
Cite this article
Zhitova, E.S., Popov, M.P., Krivovichev, S.V. et al. Quintinite-1M from the Mariinsky Deposit, Ural Emerald Mines, Central Urals, Russia. Geol. Ore Deposits 59, 745–751 (2017). https://doi.org/10.1134/S1075701517080116
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1075701517080116