Abstract
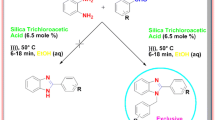
One-pot green synthesis of (2-hydroxybenzoyl)(cinnamoyl)methanes has been performed by reacting 2-hydroxyacetophenones with cinnamoyl chlorides using activated Ba(OH)2, followed by Baker–Venkataraman rearrangement assisted by dual-frequency ultrasonication. Cyclization of (2-hydroxybenzoyl)(cinnamoyl)methanes using methanesulfonic acid along with dual-frequency ultrasound irradiation resulted in the formation of 2-strylchromones in a single step. The products were obtained with high yields, and their structures were confirmed by NMR and IR spectral data.

Similar content being viewed by others
REFERENCES
Ghosal, S., Singh, S., Bhagat, M.P., and Kumar, Y., Phytochemistry, 1980, vol. 21, p. 2943. https://doi.org/10.1016/0031-9422(80)85074-6
Chemistry of Heterocyclic Compounds: Chromenes, Chromanones, and Chromones, Ellis, G.P., Ed., 1977, vol. 31, p. 455. https://doi.org/10.1002/9780470187012
Xing, Q., Liang, H., Bao, M., Li, X., Zhang, J., Bi, T., Zhang, Y., Xu, J., Du, Y., and Zhao, K., Adv. Synth. Catal., 2019, vol. 361, p. 4669. https://doi.org/10.1002/adsc.201900652
Bala, M., Kumar, S., Taxak, V.B., Boora, P., and Khatkar, S.P., J. Mater. Sci.: Mater. Electron., 2016, vol. 27, p. 9306. https://doi.org/10.1007/s10854-016-4970-y
Crouse, G.D., McGowan, M.J., and Boisvenue, R.J., J. Med. Chem., 1989, vol. 32, p. 2148. https://doi.org/10.1021/jm00129a021
Andrae, I., Bringhen, A., Böhm, F., Gonzenbach, H., Hill, T., Mulroy, L., and Truscott, T.G., J. Photochem. Photobiol., B, 1997, vol. 37, p. 147. https://doi.org/10.1016/S1011-1344(96)07330-7
Pinto, J., Silva, V.L., Silva, A.M., and Silva, A., Molecules, 2015, vol. 20, p. 11418. https://doi.org/10.3390/molecules200611418
Doria, G., Romeo, C., Forgione, A., Sberze, P., Tibolla, N., Corno, M.L., Cruzzola, G., and Cadelli, G., Eur. J. Med. Chem., 1979, vol. 14, p. 347.
Gerwick, W.H., J. Nat. Prod., 1989, vol. 52, no. 2, p. 252. https://doi.org/10.1021/np50062a005
Gerwick, W.H., Lopez, A., Van Dyune, G.D., Clardy, J., Ortiz, W., and Baez, A., Tetrahedron Lett., 1986, vol. 27, p. 1979. https://doi.org/10.1016/s0040-4039(00)84426-3
Shaw, A.Y., Chang, C.Y., Liau, H.H., Lu, P.J., Chen, H.L., Yang, C.N., and Li, H.Y., Eur. J. Med. Chem., 2009, vol. 44, p. 2552. https://doi.org/10.1016/j.ejmech.2009.01.034
Gomes, A., Fernandes, E., Silva, A.M.S., Santos, C.M.M., Pinto, D.C.G.A., Cavaleiro, J.A.S., and Lima, J.L., Bioorg. Med. Chem., 2007, vol. 15, no. 18, p. 6027. https://doi.org/10.1016/j.bmc.2007.06.046
Gomes, A., Fernandes, E., Silva, A.M.S., Pinto, D.C.G.A., Santos, C.M.M., Cavaleiro, J.A.S., and Lima, J.L.F.C., Biochem. Pharmacol., 2009, vol. 78, no. 2, p. 171. https://doi.org/10.1016/j.bcp.2009.03.028
Desideri, N., Mastromarino, P., and Conti, C., Antiviral Chem. Chemother., 2003, vol. 14, no. 4, p. 195. https://doi.org/10.1177/095632020301400404
Santos, C.M., Proença, C., Freitas, M., Araújo, A.N., Silva, A., and Fernandes, E., Abstracts of Papers, Bioheterocycles 2019—XVIII Int. Conf. on Heterocycles in Bioorganic Chemistry, 2019, p. 68.
Takao, K., Endo, S., Nagai, J., Kamauchi, H., Takemura, Y., Uesawa, Y., and Sugita, Y., Bioorg. Chem., 2019, vol. 92, article ID 103285. https://doi.org/10.1016/j.bioorg.2019.103285
Dunne, A.T.M., Gowan, J.E., Keane, J., O’Kelly, B.M., O’Sullivan, D., Roche, M.M., Ryan, P.M., and Wheeler, T.S., J. Chem. Soc., 1950, p. 1252. https://doi.org/10.1039/JR9500001252
Mahal, H.S. and Venkataraman, K., J. Chem. Soc., 1934, vol. 387, p. 1767. https://doi.org/10.1039/JR9340001767
Kumar, A. and Makrandi, J.K., Heterocycl. Lett., 2012, vol. 2, p. 271.
Pinto, D.C., Silva, A.M., and Cavaleiro, J.A., New J. Chem., 2000, vol. 24, p. 85. https://doi.org/10.1039/A908539D
Sharma, D., Kumar, S., and Makrandi, J.K., Green Chem. Lett. Rev., 2009, vol. 2, p. 53. https://doi.org/10.1080/17518250903002343
Königs, P., Neumann, O., Kataeva, O., Schnakenburg, G., and Waldvogel, S.R., Eur. J. Org. Chem., 2010, vol. 33, p. 6417. https://doi.org/10.1002/ejoc.201000957
Pinto, D.C., Silva, A.M., and Cavaleiro, J.A., Synlett, 2007, vol. 12, p. 1897. https://doi.org/10.1055/s-2007-984525
St-Gelais, A., Alsarraf, J., Legault, J., Gauthier, C., and Pichette, A., Org. Lett., 2018, vol. 20, p. 7424. https://doi.org/10.1021/acs.orglett.8b03148
Goel, S., Ritu, and Makrandi, J.K., Indian J. Chem., Sect. B, 2006, vol. 45, p. 1278. http://nopr.niscair.res.in/handle/123456789/30698
Makrandi, J.K. and Kumari, V., Synth. Commun., 1989, vol. 19, p. 1919. https://doi.org/10.1080/00397918908052583
Pinto, D.C., Silva, A.M., Almeida, L.M., Cavaleiro, J.A., Lévai, A., and Patonay, T., J. Heterocycl. Chem., 1998, vol. 35, no. 1, p. 217. https://doi.org/10.1002/jhet.5570350140
Sharma, D. and Makrandi, J.K., Green Chem. Lett. Rev., 2009, vol. 2, no. 3, p. 157. https://doi.org/10.1080/17518250903241966
Makrandi, J.K. and Seema, Chem. Ind., 1989, p. 607.
Makrandi, J.K. and Seema, Indian J. Chem., Sect. B, 1991, vol. 30, p. 788.
Mirkhani, V., Moghadam, M., Tangestaninejad, S., Mohammadpoor-Baltork, I., and Mahdavi, M., Monatsh. Chem., 2009, vol. 140, p. 1489. https://doi.org/10.1007/s00706-009-0213-8
Sadjadi, S. and Sepehrian, H., Ultrason. Sonochem., 2011, vol. 18, p. 480. https://doi.org/10.1016/j.ultsonch.2010.08.004
Feng, H., Ying, X., Peng, Y., Van der Eycken, E.V., Liu, C., Zhao, S., and Song, G., Monatsh. Chem., 2013, vol. 144, p. 681. https://doi.org/10.1007/s00706-012-0846-x
Zeng, H., Li, H., and Shao, H., Ultrason. Sonochem., 2009, vol. 16, p. 758. https://doi.org/10.1016/j.ultsonch.2009.03.008
Kumar, S., Chem. Sci. Trans., 2015, vol. 4, p. 258. https://doi.org/10.7598/cst2015.978
Kumar, S. and Sharma, D.K., Green Process. Synth., 2017, vol. 6, no. 1, p. 73. https://doi.org/10.1515/gps-2016-0099
Sasidharan, S., Raj, S., Sonawane, S., Pinjari, D., Pandit, A.B., and Saudagar, P., Nanomater. Synth., 2019, p. 27. https://doi.org/10.1016/B978-0-12-815751-0.00002-X
Salvador, R.A.J., Sa e Melo, L.M., Neves, S.A., and Campos, A.S., Tetrahedron Lett., 1993, vol. 34, p. 357. https://doi.org/10.1016/S0040-4039(00)60587-7
Zhang, Z., Zha, Z., Gan, C., Pan, C., Zhou, Y., Wang, Z., and Zhou, M.M., J. Org. Chem., 2006, vol.71, p. 4339. https://doi.org/10.1021/jo060372b
Tiwari, B.K., TrAC, Trends Anal. Chem., 2015, vol. 71, p. 100. https://doi.org/10.1016/j.trac.2015.04.013
Baig, R.B.N. and Varma, R.S., Chem. Soc. Rev., 2012, vol. 41, p. 1559. https://doi.org/10.1039/C1CS15204A
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest is declared by the authors.
Rights and permissions
About this article
Cite this article
Vashisth, N., Sharma, S.P., Kumar, S. et al. One-Pot Green Synthesis of 2-Hydroxybenzoyl(cinnamoyl)methanes and 2-Styrylchromones Using Dual-Frequency Ultrasonication. Russ J Org Chem 56, 2143–2147 (2020). https://doi.org/10.1134/S1070428020120155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020120155