Abstract
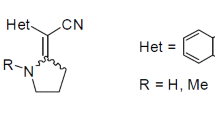
Three-component reaction of methyl 4-aryl-2,4-dioxobutanoates with aromatic aldehyde and 2-aminoacetonitrile sulfate in glacial acetic acid in the presence of anhydrous sodium acetate afforded previously unknown (3-aroyl-2-aryl-4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrol-1-yl)acetonitriles. The reaction involved intermediate formation of Schiff base, followed by Michael-type addition of the dioxo ester to the C=N bond and cyclization of the addition product, methyl 3-aroyl-4-aryl-4-(cyanomethylamino)-2-oxobutanoate. The cyclization product reacted with hydrazine hydrate at the aroyl carbonyl group to give the corresponding hydrazone which was converted in 1,4-dioxane to the cyclic form, [3,4-diaryl-6a-hydroxy-6-oxo-3a,4,6,6a-tetrahydropyrrolo[3,4-c]pyrazol-5(1H)-yl]acetonitrile, without elimination of the second water molecule. When the reaction of (3-aroyl-2-aryl-4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrol-1-yl)acetomtriles with hydrazine hydrate was carried out in boiling acetic acid, [3,4-diaryl-6-oxo-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]acetonitriles were obtained as a result of dehydration of initially formed [3,4-diaryl-6a-hydroxy-6-oxo-3a,4,6,6a-tetrahydropyrrolo[3,4-c]pyrazol-5(1H)-yl]acetonitriles as shown by special experiment. The structures of [6a-hydroxy-6-oxo-3,4-diphenyl-3a,4,6,6a-tetrahydropyrrolo[3,4-c]pyrazol-5(1H)-yl]acetonitrile and [4-(4-methoxyphenyl)-6-oxo-3-phenyl-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]acetonitrile were determined by X-ray analysis.
Similar content being viewed by others
References
Mashkovskii, M.D., Lekarstvennye sredstva (Medicines), Moscow: Novaya Volna, 2008, 15th ed., p. 826.
Berestovitskaya, V.M., Tyurenkov, I.N., Vasil’eva, O.S., Perfilova, V.N., Ostroglyadov, E.S., and Bagmetova, V.V., Racetamy: metody sinteza i biologicheskaya aktivnost’ (Racetams: Methods of Synthesis and Biological Activity), St. Petersburg: Asterion, 2016, vol. 1, p. 287.
Barcelo, VS. and Bienz, S., J. Org. Chem., 2018, vol. 83, p. 2734. doi https://doi.org/10.1021/acs.joc.7b03187
Gein, V.L., Buldakova, E.A., Korol, A.N., Veihman, G.A., and Dmitriev, M.V., Russ. J. Gen. Chem., 2018, vol. 88, p. 908. doi https://doi.org/10.1134/S1070363218050110
Bellamy, L.J., The Infra-red Spectra of Complex Molecules, London: Methuen, 1958. Translated under the title Infrakrasnye spektry slozhnykh molekul, Moscow: Inostrannaya Literatura, 1963, p. 381.
Kazitsina, L.A. and Kupletskaya, N.B., Primenenie IK, UF, YaMR-spektroskopii v organicheskoi khimii (Application of IR, UV, and NMR Spectroscopy in Organic Chemistry), Moscow: Vysshaya Shkola, 1971, pp. 33, 42.
Andreichikov, Yu.S., Gein, V.L., and Anikina, I.N., Zh. Org. Khim., 1986, vol. 22, p. 1749.
CrysAlisPro, Version 1.171.37.33 (release 27-03-2014 CrysAlis171.NET), Agilent Technologies.
Sheldrick, G.M., Acta Crystallogr., Sect. A, 2008, vol. 64, p. 112. doi https://doi.org/10.1107/S01087673007043930
Sheldrick, G.M., Acta Crystallogr., Sect. C, 2015, vol. 71, p. 3. doi https://doi.org/10.1107/S2053229614024218
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J, Howard, J.A.K., and Puschmann, H., J. Appl. Crystallogr., 2009, vol. 42, p. 339. doi https://doi.org/10.1107/S0021889808042726
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interests
The authors declare the absence of conflict of interests.
Russian Text © The Author(s), 2019, published in Zhurnal Organicheskoi Khimii, 2019, Vol. 55, No. 7, pp. 1046–1054.
Rights and permissions
About this article
Cite this article
Gein, V.L., Buldakova, E.A. & Dmitriev, M.V. Synthesis of (3-Aroyl-2-aryl-4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrol-1-yl)acetonitriles and Their Reaction with Hydrazine Hydrate. Russ J Org Chem 55, 951–957 (2019). https://doi.org/10.1134/S1070428019070054
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428019070054
Keywords
- three-component reaction
- (3-aroyl-2-aryl-4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrol-1-yl)acetonitriles
- 2-aminoacetonitrile sulfate
- hydrazine hydrate
- [3,4-diaryl-6a-hydroxy-6-oxo-3a,4,6,6a-tetrahydropyrrolo[3,4-c]pyrazol-5(1H)-yl]acetonitriles
- [3,4-diaryl-6-oxo-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]-acetonitriles