Abstract
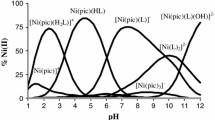
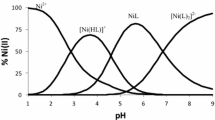
The formation of nickel citrate complexes was studied at ionic strength values of 0.1 and 0.3 mol/l (Et4NCl) and 298.15 K by potentiometric titration. The NiCit−, NiHCit, and NiH2Cit+ complexes were formed in a Ni2+ ion-citric acid (H3Cit) system. The thermodynamic formation constants of the nickel(II) citrate complexes were calculated in an aqueous solution at \(I = 0:\log \beta _{NiCit^ - }^0 \) = 6.86 ± 0.12 (Ni2+ + Cit3− ai NiCit−), logK 01 = 4.18 ± 0.10 (Ni2+ + HCit2− ai NiHCit), and logK 02 = 2.24 ± 0.11 (Ni2+ + H2Cit− ai NiH2Cit+). The spectral properties of the Ni2+-H3Cit system were studied by spectrometry. The conditions of calorimetric determination of the thermal effects of formation of the nickel citrate complexes in an aqueous solution were optimized on the basis of the calculated stability constants of the Ni(II) complexes with H3Cit.
Similar content being viewed by others
References
Daniele, P.G., Ostacoli, G., Rigano, C., and Sammartano, S., Transition Met. Chem., 1984, vol. 9, no. 10, p. 385.
Hedwin, G., Liddle, J., and Reeves, R., Aust. J. Chem., 1980, vol. 33, p. 1685.
Daniele, P.G., Ostacoli, G., and Amico, P., Talanta, 1978, vol. 25, no. 3, p. 177.
Daniele, P. and Ostacoli, G., Ann. Chim. (Rome), 1976, vol. 66, p. 537.
Li, N., Lindenbaum, A., and White, J., J. Inorg. Nucl. Chem., 1959, vol. 12, nos. 1–2, p. 122.
Patnaik, R. and Pani, S., J. Indian Chem. Soc., 1965, vol. 42, p. 527.
Campi, E., Ostacoli, G., Meirone, M., and Saini, G., J. Inorg. Nucl. Chem., 1964, vol. 26, no. 4, p. 553.
Patnaik, R. and Pani, S., J. Indian Chem. Soc., 1957, vol. 34, p. 619.
Patra, S. and Pani, S., J. Indian Chem. Soc., 1957, vol. 32, p. 572.
Vasil’ev, V.P., Morozova, R.P., and Kochergina, L.A., Praktikum po analiticheskoi khimii (Practical Works in Analytical Chemistry), Moscow: Khimiya, 2000.
Kozlovskii, E.V., Doctoral (Chem.) Dissertation, Ivanovo: Inst. of the Chemistry of Nonaqueous Solutions of the RAS, 1995.
Borodin, V.A., Kozlovskii, E.V., and Vasil’ev, V.P., Zh. Neorg. Khim., 1986, vol. 31, no. 1, p. 10.
Vasil’ev, V.P., Chernikov, V.V., and Golubeva, T.E., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 2001, no. 1, p. 14.
Nazarenko, V.A., Antonovich, V.P., and Nevskaya, E.M., Gidroliz ionov metallov v razbavlennykh rastvorakh (Hydrolysis of Metal Ions in Dilute Solutions), Moscow: Atomizdat, 1979.
Davies, C.W., J. Chem. Soc., 1938, p. 2093.
Vasil’ev, V.P., Teor. Eksp. Khim., 1966, no. 2, p. 353.
Zelenin, O.Yu., Kochergina, L.A., and Vasil’ev, V.P., Zh. Fiz. Khim., 2004, vol. 78, no. 7, p. 1245 [Russ. J. Phys. Chem. (Engl. Transl.), vol. 78, no. 7, p. 1082].
Author information
Authors and Affiliations
Additional information
Original Russian Text © O.Yu. Zelenin, 2007, published in Koordinatsionnaya Khimiya, 2007, Vol. 33, No. 5, pp. 355–359.
Rights and permissions
About this article
Cite this article
Zelenin, O.Y. Interaction of the Ni2+ ion with citric acid in an aqueous solution. Russ J Coord Chem 33, 346–350 (2007). https://doi.org/10.1134/S1070328407050065
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1070328407050065