Abstract
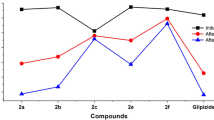
A series of amides based on (2S)-cyanopyrrolidine and α, β-unsaturated aryl- and hetarylcarboxylic acids have been synthesized. The dependence of the hypoglycemic activity of compounds on the structure of the aromatic fragment has been studied in the oral glucose tolerance test in mice. Amides based on (E)-3-phenylprop-2-enoic and (E)-3-(4-methoxyphenyl)prop-2-enoic acids and (2S)-cyanopyrrolidine have been shown to significantly reduce blood glucose levels in mice. The observed hypoglycemic effect at a dose of 10 mg/kg is comparable to the effect of hypoglycemic drug vildagliptin.


Similar content being viewed by others
REFERENCES
WHO: 10 Facts on Diabetes, World Health Organization, 2016. http://www.who.int/features/factfiles/diabetes/en/. Accessed May 17, 2017.
Thornberry, N.A. and Gallwitz, B., Best Pract. Res., Clin. Endocrinol. Metab., 2009, vol. 23, pp. 479–486.
Nabeno, M., Akahoshi, F., Kishida, H., Miyaguchi, I., Tanaka, Y., Ishii, S., and Kadowaki, T., Biochem. Biophys. Res. Commun., 2013, vol. 434, pp. 191–196.
Spasov, A.A., Popov, Yu.V., Lobasenko, V.S., Korchagina, T.K., Vassiliev, P.M., Kuznetsova, V.A., Brigadirova, A.A., Rashchenko, A.I., Babkov, D.A., Kochetkov, A.N., Kovaleva, A.I., and Efremova, O.S., Russ. J. Bioorg. Chem., 2017, vol. 43, pp. 163–169.
Spasov, A.A., Vasil’ev, P.M., Babkov, D.A., Prokhorova, T.Y., Sturova, E.A., Klimochkin, Y.N., Leonova, M.V., and Baimuratov, M.R., Russ. J. Bioorg. Chem., 2017, vol. 43, pp. 449–455.
Li, N., Wang, L.-J., Jiang, B., Guo, S.-J., Li, X.-Q., Chen, X.-C., Jiao, L., Li, C., Wang, Y., and Shi, D.-Y., Bioorg. Med. Chem. Lett. Pergamon, 2018, vol. 28, pp. 2131–2135.
Newman, D.J. and Cragg, G.M., J. Nat. Prod., 2007, vol. 70, pp. 461–477.
Gao, Y., Zhang, Y., Zhu, J., Li, B., Li, Z., Zhu, W., Shi, J., Jia, Q., and Li, Y., Future Med. Chem., 2015, vol. 7, pp. 1079–1089.
Sova, M., Mini Rev. Med. Chem., 2012, vol. 12, pp. 749–767.
Kepa, M., Miklasinska-Majdanik, M., Wojtyczka, R.D., Idzik, D., Korzeniowski, K., Smolen-Dzirba, J., and Wasik, T.J., Biomed Res. Int. Hindawi, 2018, vol. 2018, pp. 1–9.
Da, Cunha F.M., Duma, D., Assreuy, J., Buzzi, F.C., Niero, R., Campos, M.M., and Calixto, J.B., Free Radic. Res., 2004, vol. 38, pp. 1241–1253.
Avanesyan, A.A., Pashkov, A.N., Simonyan, N.A., Simonyan, A.V., and Myachina, O.V., Pharm. Chem. J., 2009, vol. 43, pp. 249–250.
Huggins, D.J., Sherman, W., and Tidor, B., J. Med. Chem. Am. Chem. Soc., 2012, vol. 55, pp. 1424–1444.
Jha, V. and Bhadoriya, K., S, J. Mol. Struct. Elsevier, 2018, vol. 1158, pp. 96–105.
Patent WO 2014/105926 A1, 2014.
Pace, P., Francesco, E.D., Gardelli, C., Harper, S., Muraglia, E., Nizi, E., Orvieto, F., Petrocchi, A., Poma, M., Rowley, M., Scarpelli, R., Laufer, R., Paz, O.G., Monteagudo, E., Bonelli, F., Hazuda, D., Stillmock, K.A., and Summa, V., J. Med. Chem. Am. Chem. Soc., 2007, vol. 50, pp. 2225–2239.
Wang, Z., Wei, P., Xizhi, X., Liu, Y., Wang, L., and Wang, Q., J. Agric. Food Chem. Am. Chem. Soc., 2012, vol. 60, pp. 8544–8551.
Wang, J., Feng, Y., Ji, X., Deng, G., Leng, Y., and Liu, H., Bioorg. Med. Chem. Pergamon, 2013, vol. 21, pp. 7418–7429.
Pawar, H.S., Wagh, A.S., and Lali, A.M., New J. Chem. R. Soc. Chem., 2016, vol. 40, pp. 4962–4968.
Robbins, R.J. and Schmidt, W.F., J. Label. Compd. Radiopharm. Wiley, 2004, vol. 47, pp. 797–806.
Patent CN 101417969 A, 2009.
Aitken, R.A., Smith, M.H., and Wilson, H.S., J. Mol. Struct. Elsevier, 2016, vol. 1113, pp. 171–173.
White, J.R., Clin. Diabetes. Am. Diabetes Assoc., 2008, vol. 26, pp. 53–57.
Gribble, F.M., Williams, L., Simpson, A.K., and Reimann, F., Diabetes, 2003, vol. 52, pp. 1147–1154.
Armarego, W.L.F. and Perrin, D.D., Purification of Laboratory Chemicals, Butterworth Heinemann, 1997.
ACKNOWLEDGMENTS
The authors thank the Chemical Research Center for Collective Use of the Siberian Branch of the Russian Academy of Sciences for carrying out spectral and analytical measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest
The authors state that there is no conflict of interests.
Statement on the Welfare of Animals
All applicable international, national and institutional guidelines for the care and use of animals have been observed.
Additional information
Translated by A. Levina
Abbreviations: GTT, glucose tolerance test; DPP-4, dipeptidyl peptidase-4; DM-2, type 2 diabetes mellitus; GLP-1, glucagon-like peptide-1; GIP, glucose-dependent insulinotropic polypeptide.
Corresponding author: fax: (383) 330 9752; e-mail: s.o.kuranov@chemomsu.ru.
Rights and permissions
About this article
Cite this article
Kuranov, S.O., Blokhin, M.E., Borisov, S.A. et al. Synthesis and Hypoglycemic Activity of Aryl(Hetaryl)Propenoic Cyanopyrrolidine Amides. Russ J Bioorg Chem 45, 374–380 (2019). https://doi.org/10.1134/S1068162019050078
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162019050078