Abstract
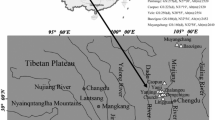
Body size variation across environmental gradients has received considerable attention in evolutionary ecology in recent years. In this study, we investigated body sizes and age structure using skeletochronology in male Polypedates megacephalus from five sites with the attitude ranging from 449 to 1300 m. The results showed age at sexual maturity in males was 2 years old, and the longevity was 5 years old except for Changning population of 3 years. Body size was significantly correlated with age within each population except for Changning population. Average age did not differ significantly among populations. Body size of individuals also did not differ across populations when removing the effect of age, which did not follow Bergmann’s rule.
Similar content being viewed by others
References
Atkinson, D. and Sibly, R.M., Why are organisms usually bigger in colder environments? Making sense of a life history puzzle, Trends Ecol. Evol., 1997, vol. 12, no. 6, pp. 235–239.
Bergmann, C., Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Größe, Göttinger Stud., 1847, vol. 3, no. 1, pp. 595–708.
Ashton, K.G., Do amphibians follow Bergmann’s rule?, Can. J. Zool., 2002, vol. 80, no. 4, pp. 708–716.
Blackburn, T.M. and Hawkins, B.A., Bergmann’s rule and the mammal fauna of northern North America, Ecography, 2004, vol. 27, no. 6, pp. 715–724.
Meiri, S. and Dayan, T., On the validity of Bergmann’s rule, J. Biogeogr., 2003, vol. 30, no. 3, pp. 331–351.
Adams, D.C. and Church, J.O., Amphibians do not follow Bergmann’s rule, Evolution, 2008, vol. 62, no. 2, pp. 413–420.
Ashton, K.G., Sensitivity of intraspecific latitudinal clines of body size for tetrapods to sampling, latitude and body size, Integr. Comp. Biol., 2004, vol. 44, no. 6, pp. 403–412.
Liao, W.B. and Lu, X., Adult body size = f (initial size + growth rate × age): Explaining the proximate cause of Bergman’s cline in a toad along altitudinal gradients, Evol. Ecol., 2012, vol. 26, no. 3, pp. 579–590.
Laugen, A. T., Laurila, A., Jönsson, I., Söderman, F., and Merilä, J., Do common frogs (Rana temporaria) follow Bergmann’s rule?, Evol. Ecol. Res., 2005, vol. 7, no. 5, pp. 717–731.
Ashton, K.G. and Feldman, C.R., Bergmann’s rule in nonavian reptiles: Turtles follow it, lizards and snakes reverse it, Evolution, 2003, vol. 57, no. 5, 1151–1163.
Liao, W.B., Liu, W.C., and Merilä, J., Andrew meets Rensch: Sexual size dimorphism and the inverse of Rensch’s rule in Andrew’s toad (Bufo andrewsi), Oecologia, 2015, vol. 177, no. 2, pp. 389–399.
Conover, D.O. and Schultz, E.T., Phenotypic similarity and the evolutionary significance of countergradient variation, Trends Ecol. Evol., 1995, vol. 10, no. 6, pp. 248–252.
Mousseau, T.A., Ectotherms follow the converse to Bergmann’s rule, Evolution, 1997, vol. 51, no. 2, pp. 630–632.
Blanckenhorn, W.U. and Demont, M., Bergmann and converse Bergmann latitudinal clines in arthropods: Two ends of a continuum?, Integr. Comp. Biol., 2004, vol. 44, no. 6, pp. 413–424.
Fei, L., Ye, C.Y., and Jiang, J.P., Colored Atlas of Chinese Amphibians, Chengdu: Sichuan Publishing House of Science and Technology, 2010.
Wells, K.D., The social behaviour of anuran amphibians, Anim. Behav., 1977, vol. 25, pp. 666–693.
Liao, W.B., Lou, S.L., Zeng, Y., and Kotrschal, A., Large brains, small guts: The expensive tissue hypothesis supported in anurans, Am. Nat., 2016, vol. 188, no. 6, pp. 693–700.
Lüpold, S., Jin, L. and Liao, W.B., Population density drives differential investment in pre- and postmating sexual traits in frogs, Evolution, 2017, in press. doi 10.1111/evo.13246
Mai, C.L., Liao, J., Zhao, L., Liu, S.M., and Liao, W.B., Brain size evolution in the frog Fejervarya limnocharis does neither support the cognitive buffer nor the expensive brain framework hypothesis, J. Zool., 2016, vol. 302, no. 1. doi 10.1111/jzo.12432
Castanet, J., Meunier, F. J. and Francillon-Vieillot. H., Squelettochronologie à partir des os et des dents chez les vertébrés, in Tissus Durs et Âge Individuel des Vertébrés, Bagliniere, J.L., Castanet, J., Conad, F., and Meunier, J., Eds., Paris: Orstom-Inra Editions, 1991, pp. 257–280.
Castanet, J. and Smirina, E., Introduction to the skeletochronological method in amphibians and reptiles, Ann. Sci. Nat. Zool. Biol. Anim., 1990, vol. 11, no. 4, pp. 191–196.
Liao, W.B., Luo, Y., Lou, S.L., Lu, D., and Jehle, R., Geographic variation in life-history traits: Growth season affects age structure, egg size and clutch size in Andrew’s toad (Bufo andrewsi), Front. Zool., 2016, vol. 13, no. 6. doi 10.1186/s12983-016-0138-0
Chen, W., Yu, T.L., and Lu, X., Age and body size of Rana kukunoris, a high-elevation frog native to the Tibetan plateau, Herpetol. J., 2011, vol. 21, no. 2, pp. 149–151.
Liao, W.B., A skeletochronological estimate of age in a population of the Siberian Wood Frog, Rana amurensis, from northeastern China, Acta Herpetol., 2011, vol. 6, no. 2, pp. 237–245.
Liao, W.B. and Lu, X., Male mating success in the Omei treefrog (Rhacophorus omeimontis): The influence of body size and age, Belg. J. Zool., 2011, vol. 141, no. 2, pp. 3–12.
Liao, W.B., Lu, X., Shen, Y.W., and Hu, J.C., Age structure and body size of two populations of the rice frog Rana limnocharis from different altitudes, Ital. J. Zool., 2011, vol. 78, no. 2, pp. 215–221.
Von Bertalanffy, L., Quantitative laws in metabolism and growth, Q. Rev. Biol., 1957, vol. 32, no. 3, pp. 217–231.
Miaud, C., Ūzüm, N., Avci, A., and Olgun, K., Age, size and growth of the endemic Anatolian mountain frog Rana holtzi from Turkey, Herpetol. J., 2007, vol. 17, no. 3, pp. 167–173.
Li, S.T., Wu, X., Li, D.Y., Lou, S.L., Mi, Z.P., and Liao, W.B., Body size variation of odorous frogs (Odorrana grahami) across altitudinal gradients, Herpetol. J., 2013, vol. 23, no. 4, pp. 187–192.
Huang, Y., Zhu, H.Q., Liao, Y.M., Jin, L., and Liao, W.B., Age and body size of the toad Bombina maxima in a subtropical high-altitude population, Herpetol. J., 2013, vol. 23, no. 4 pp. 229–232.
Maria, G.F., Gerardo, G., and Franco, A., Huge but moderately long-lived: Age structure in the mountain chicken, Leptodactylus fallax, from Montserrat, West Indies, Herpetol. J., 2014, vol. 24, no. 3, pp. 167–173.
Platz, J.E., Lathrop, A., Hofbauer, L., and Vradenburg, M., Age distribution and longevity in the Ramsey Canyon leopard frog, Rana subaquavocalis, J. Herpetol., 1997, vol. 31, no. 4, pp. 552–557.
Gibbons, M.M. and McCarthy, T.K., Growth, maturation and survival of frogs Rana temporaria L., Ecography, 1984, vol. 7, no. 4, pp. 419–427.
Cherry, M.I. and Francillon-Vieillot, H., Body size, age and reproduction in the leopard toad, Bufo pardalis, J. Zool., 1992, vol. 228, no. 1, pp. 41–50.
Platz, J.E. and Lathrop, A., Body size and age assessment among advertising male chorus frogs, J. Herpetol., 1992, vol. 27, pp. 109–111.
Liu, Y.H., Zeng, Y., Liao, W.B., Zhou, C.Q., Mi, Z.P., Mao, M., and Chen, L., Altitudinal variation in body size in the Rice Frog (Rana limnocharis) in southwestern China, Acta Herpetol., 2012, vol. 7, no. 1, pp. 57–68.
Berven, K, A. and Gill, D.E., Interpreting geographic variation in life-history traits, Am. Zool., 1983, vol. 23, pp. 8597.
Brown, J.H., Gillooly, J.F., Allen, A.P., Savage, V.M., and West, G.B., Toward a metabolic theory of ecology, Ecology, 2004, vol. 85, pp. 1771–1789.
Matthews, K.R. and Miaud, C., A skeletochronological study of the age structure, growth, and longevity of the mountain yellow-legged frog, Rana muscosa, in the Sierra Nevada, California, Copeia, 2007, vol. 2007, no. 4, pp. 986–993.
Liao, W.B. and Lu, X., A skeletochronological estimation of age and body size by the Sichuan torrent frog (Amolops mantzorum) between two populations at different altitudes, Anim. Biol., 2010, vol. 60, no. 4, pp. 479–489.
Berven, K.A., The genetic basis of altitudinal variation in the wood frog Rana sylvatica: 1. An experimental analysis of life history traits, Evolution, 1982, vol. 36, no. 5, pp. 962–983.
Liao, W.B. and Lu, X., Age structure and body size of the Chuanxi Tree Frog Hyla annectans chuanxiensis from two different elevations in Sichuan (China), Zool. Anz., 2010, vol. 248, no. 4, pp. 255–263.
Liao, W.B. and Lu, X., Variation in mating patterns in the Andrew’s toad Bufo andrewsi along an elevational gradient in southwestern China, Ethol. Ecol. Evol., 2012, vol. 24, no. 2, pp. 174–186.
Ashton, K.G., Patterns of within species body size variation of birds: Strong evidence for Bergmann’s rule, Global Ecol. Biogeogr., 2002, vol. 11, no. 6, pp. 505–523.
Olalla-Tárraga, M.Á. and Rodríguez, M.Á., Energy and interspecific body size patterns of amphibian faunas in Europe and North America: Anurans follow Bergmann’s rule, urodeles its converse, Global Ecol. Biogeogr., 2007, vol. 16, no. 5, pp. 606–617.
Liao, W.B. and Lu, X., Variation in body size, age and growth in the Omei Treefrog (Rhacophorus omeimontis) along an altitudinal gradient in western China, Ethol. Ecol. Evol., 2011, vol. 23, no. 3, pp. 248–261.
Lou, S.L., Jin, L., Liu, Y.H., Mi, Z.P., Tao, G., Tang, Y.M., and Liao, W.B., Altitudinal variation in age and body size in Yunnan Pond Frog (Pelophylax pleuraden), Zool. Sci., 2012, vol. 29, no. 8, pp. 493–498.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Jin, L., Chen, C. & Liao, W.B. Altitudinal variation in body size and age in male spot-legged treefrog (Polypedates megacephalus). Russ J Ecol 48, 476–481 (2017). https://doi.org/10.1134/S1067413617050083
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1067413617050083