Abstract
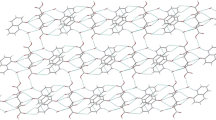
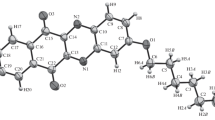
4-(Piperidyl-1)-2-phenylpyrido[2,3-a]anthraquinone-7,12 monobromohydrate (HL)Br · 3H2O (I) and 4-(piperidyl-1)-2-phenylpyrido[2,3-a]anthraquinone-7,12 dibromohydrate (H2 L)Br2 · 3H2O (II) are isolated in the crystalline state. The crystal structures of compounds I and II are determined using X-ray diffraction. It is established that the protonation of 4-(piperidyl-1)-2-phenylpyrido[2,3-a]anthraquinone-7,12 proceeds primarily through the pyridine atom at pH 2–3. The attachment of the second proton occurs through the piperidine nitrogen atom at pH ∼ 1.
Similar content being viewed by others
References
B. I. Stepanov, An Introduction to the Chemistry and Technology of Organic Dyes (Khimiya, Moscow, 1984) [in Russian].
V. Ya. Faĭn, 9,10-Anthraquinones and Their Application (Photochemistry Center, Russian Academy of Sciences, Moscow, 1999) [in Russian].
V. Ya. Faĭn, Electronic Absorption Spectra and the Structure of 9,10-Anthraquinones (Sputnik+, Moscow, 2003), Vol. 1 [in Russian].
R. A. Muzychkina, Natural Anthraquinones: Biological Properties and Physicochemical Characteristics (Fazis, Moscow, 1998) [in Russian].
S. B. Strashnova, B. E. Zaitsev, V. E. Zavodnik, O. V. Kovalchukova, and D. P. Voronin, Koord. Khim. 33(11), 864 (2007) [Russ. J. Coord. Chem. 33 (11), 850 (2007)].
V. V. Davydov, M. G. Sarabiya, A. I. Ezhov, et al., Koord. Khim. 20(10), 803 (1994).
G. M. Sheldrick, SHELX97: Program for the Solution and Refinement of Crystal Structures (University of Göttingen, Göttingen, Germany, 1997).
O. V. Kovalchukova, A. I. Stash, S. B. Strashnova, V. K. Belsky, Tran Than Tung, and B. E. Zaĭtsev, Kristallografiya 53(2), 270 (2008) [Crystallogr. Rep. 53 (3), 451 (2008)].
V. I. Minkin, B. Ya. Simkin, and R. M. Minyaev, Theory of Molecular Structure (Feniks, Rostov-on-Don, 1977) [in Russian].
M. Dewar, The Molecular Orbital Theory of Organic Chemistry (McGraw-Hill, New York, 1969; Mir, Moscow, 1973).
N. E. Kuz’mina, K. K. Palkina, N. V. Polyakova, I. N. Golubev, A. N. Medvedev, O. V. Koval’chukova, S. B. Strashnova, B. E. Zaitsev, A. N. Levov, and F. Toze, Zh. Neorg. Khim. 47(11), 1843 (2002) [Russ. J. Inorg. Chem. 47 (11), 1693 (2002)].
N. E. Kuz’mina, K. K. Palkina, N. V. Polyakova, I. N. Golubev, A. N. Medvedev, O. V. Koval’chukova, S. B. Strashnova, N. I. Mordovina, S. V. Nikitin, and B. E. Zaitsev, Zh. Neorg. Khim. 48(2), 257 (2003) [Russ. J. Inorg. Chem. 48 (2), 205 (2003)].
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.V. Kovalchukova, A.I. Stash, V.K. Belsky, S.B. Strashnova, B.E. Zaĭtsev, M.A. Ryabov, 2009, published in Kristallografiya, 2009, Vol. 54, No. 1, pp. 72–76.
Rights and permissions
About this article
Cite this article
Kovalchukova, O.V., Stash, A.I., Belsky, V.K. et al. Synthesis and the crystal and molecular structures of 4-(piperidyl-1)-2-phenylpyrido[2,3-a]anthraquinone-7,12 Mono- and dibromohydrates (HL)Br · 3H2O and (H2 L)Br2 · 3H2O. Crystallogr. Rep. 54, 68–73 (2009). https://doi.org/10.1134/S106377450901012X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106377450901012X