Abstract
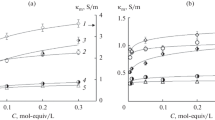
The current efficiencies of the water dissociation water and the voltage-current characteristics of the bipolar (asymmetric bipolar) membranes were measured in a two-chamber electrochemical cell. The cell was formed of an MB-3 bipolar membrane or an asymmetric bipolar membrane, which is an MA-40 heterogeneous membrane with a thin surface layer in the form of a cation-selective homogeneous film and MA-40 and MA-41 heterogeneous monopolar membranes. The dissociation of water on MA-40 in 0.01 M sodium chloride decreased the current efficiency of the acid and alkali both in the channel with a bipolar membrane and in the channel with an asymmetric bipolar membrane. The effective ion transport numbers across MA-40 and MA-41 at different pH values were determined. The water dissociation rate on MA-40 decreased at pH > 9.5. A kinetic model of the electrodialysis of a dilute solution of sodium chloride in a two-chamber unit cell with a bipolar and anionite membranes was suggested.
Similar content being viewed by others
References
Frilette, V., J. Phys. Chem., 1956, vol. 60, p. 435.
Greben’, V.P., Mel’nik, N.M., and Nechunaev, V.P., in Neorganicheskie resursy morya (Inorganic Resources of the Sea), Vladivostok: Dal’nevost. Nauchn. Tsentr Akad. Nauk SSSR, 1978, p. 126.
Greben’, V.P., Kozlova-Markova, K.N., and Kornienko, I.I., in Elektrokhimiya ionitov. Nauch. tr. Kuban. gos. un-ta (Ionite Electrochemistry: Transactions of Kuban State University), Krasnodar, 1979, p. 45.
Bobrinskaya, G.A., Pavlova, T.V., and Shatalov, A.Ya., Zh. Prikl. Khim., 1985, vol. 58, p. 786.
Pourcelly, G. and Gavach, C., Handbook on Bipolar Membrane Technology, Kemperman, A.J.B., Ed., Twente: Univ. Press., 2000, p. 17.
Xu, T.W., Desalination, 2001, vol. 140, p. 247.
Bobrinskaya, G.A., Mikhaleva, G.N., and Shatalov, A.Ya., Khim. Tekhnol. Vody, 1985, vol. 7, p. 62.
Grabowski, A., Zhang, G., Strathmann, H., and Eigenberger, G., Separation and Purification Technology, 2008, vol. 60, p. 86.
Vera, E., Sandeaux, J., Persin, F., Pourcelly, G., Dornier, M., and Ruales, J., J. Food Eng., 2007, vol. 78, p. 1435.
Balster, J., Punt, J.I., Stamatialis, D.F., Lammers, H., Verver, A.B., and Wessling, M., J. Membr. Sci., 2007, vol. 303, p. 213.
Pourcelly, G. and Bazinet, L., New York: CRC Press, Taylor & Francis Group, 2009, p. 581.
Greben’, V.P., Pivovarov, N.Ya., and Latskov, V.L., Zh. Prikl. Khim., 1988, vol. 61, p. 990.
Greben’, V.P., Pivovarov, N.Ya., Chetverikova, A.T., and Rodzik, I.G., Zh. Prikl. Khim., 1993, vol. 66, p. 574.
Zabolotskii, V.I., Shel’deshov, N.V., Illarionova, V.M., Kovarskii, N.Ya., Greben’, V.P., and Pivovarov, N.Ya., USSR Inventor’s Certificate no. 2 923 912, 1981.
Greben’, V.P., Pivovarov, N.Ya., Latskov, V.L., Rodzik, I.G., Kovarskii, N.Ya, Gnusin, N.P., Zabolotskii, V.I., Shel’deshov, N.V., and Lebedev, V.Yu., USSR Inventor’s Certificate no. 3 736 129, 1986.
Greben’, V.P., Pivovarov, N.Ya., Latskov, V.L., Rodzik, I.G., Kovarskii, N.Ya., Gnusin, N.P., Zabolotskii, V.I., Shel’deshov, N.V., and Lebedev, V.Yu., USSR Inventor’s Certificate no. 3 736 135, 1986.
Wisniewski, J., Wisniewska, G., and Winnicki, T., Desalination, 2004, vol. 169, p. 11.
Shel’deshov, N.V., Zabolotskii, V.I., Ganych, V.V., Shadrina, M.V., Shmal’ts, L.S., Balavadze, E.M., and Mekvabishvili, T.V., IV Vsesoyuz. konf. “Membranosorbtsionnye protsessy razdeleniya veshchestv i ikh primenenie v narodnom khozyaistve,” Cherkassy, 1988, p. 132.
Paleologou, M., Thibault, A., Wong, P.-Y., Thompson, R., and Berry, R.M., Separation and Purification Technology, 1997, vol. 11, p. 159.
Zabolotskii, V.I., El’nikova, L.F., Shel’deshov, N.V., and Alekseev, A.V., Elektrokhimiya, 1987, vol. 23, p. 1626.
Wilson, J.R., Demineralisation by Elektrodialysis, London: Butterworth Scientific Publications, 1960.
Greben’, V.P., Pivovarov, N.Ya., Kovarskii, N.Ya., and Nefedova, G.Z., Zh. Fiz. Khim., 1978, vol. 52, p. 2641.
Simons, R., Nature, 1979, vol. 280, p. 824.
Timashev, S.F. and Kirganova, E.V., Elektrokhimiya, 1981, vol. 17, p. 440.
Zabolotskii, V.I., Gnusin, N.P., and Shel’deshov, N.V., Elektrokhimiya, 1984, vol. 20, p. 1340.
Shel’deshov, N.V., Zabolotskii, V.I., Pis’menskaya, N.D., Gnusin, N.P., and Shel’deshov, N.V., Elektrokhimiya, 1986, vol. 22, p. 791.
Zabolotskii, V.I., Shel’deshov, N.V., and Gnusin, N.P., Usp. Khim., 1988, vol. 57, p. 1403.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.I. Zabolotskii, S.V. Utin, N.V. Shel’deshov, K.A. Lebedev, P.A. Vasilenko, 2011, published in Elektrokhimiya, 2011, Vol. 47, No. 3, pp. 343–348.
Rights and permissions
About this article
Cite this article
Zabolotskii, V.I., Utin, S.V., Shel’deshov, N.V. et al. Correction of pH of diluted solutions of electrolytes by electrodialysis with bipolar membranes. Russ J Electrochem 47, 321–326 (2011). https://doi.org/10.1134/S1023193511030141
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193511030141