Abstract
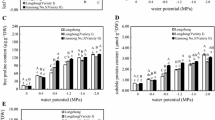
This study examines the effects of humic acid (HA, control, 3 and 6 mg/L) on some biochemical and physiological parameters of rapeseed (Brassica napus L.) plants under different water supply conditions (60, 100, and 140 mm evaporation from class A pan). Water stress decreased chlorophyll a (Chl a) and total chlorophyll (ChlT) content in plants but proline content partly increased with increasing water stress severity. Plants treated by HA had more Chl a and ChlT content under both well and limited water conditions. Application of HA improved the PSII and peroxidase activity of rapeseed plants under all irrigation treatments. Ascorbate peroxidase activity under severe water stress condition increased by 70 and 95%, compared with that under moderate and well watering conditions, respectively. Catalase activity was 51 and 69% less under well watering than that of moderate and severe water stress conditions, respectively. The highest activity of ascorbate peroxidase was recorded in plants treated by 6 mg/L HA. HA-treated plants had 42, 8.5, and 15% more soluble protein content under well watering, moderate and severe water stress conditions, respectively, compared with control plants. Malondialdehyde increased with increasing the severity of water stress, in contrast, application of HA significantly reduced the amount of this trait under water stress conditions. It was shown that application of HA increased the activity of antioxidant enzymes, improved PSII activity and consequently decreased lipid peroxidation in rapeseed plants.
Similar content being viewed by others
Abbreviations
- APX:
-
ascorbate peroxidase
- CAT:
-
catalase
- Chl a :
-
chlorophyll a
- ChlT :
-
total chlorophyll
- HA:
-
humic acid
- POD:
-
peroxidase
- MWS:
-
moderate water stress
- SWS:
-
severe water stress
- WWC:
-
well watering conditions
References
Manivannan, P., Abdul-Jaleel, C., Somasundaram, R., and Panneerselvam, R., Osmoregulation and antioxidant metabolism in drought-stressed Helianthus annuus under triadimefon drenching, C. R. Biol., 2008, vol. 331, pp. 418–425.
Diego, A.M., Oliva, M.A., Carlos, A.M., and Cambraia, J., Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress, Environ. Exp. Bot., 2003, vol. 49, pp. 69–76.
Bian, S. and Jiang, Y., Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery, Sci. Hortic., 2009, vol. 120, pp. 264–270.
Bray, E.A., Plant responses to water deficit, Trends Plant Sci., 1997, vol. 2, pp. 48–54.
Sinha, S., Saxena, R., and Singh, S., Chromium induced lipid peroxidation in the plants of Pistia stratiotes L. role of antioxidants and antioxidant enzymes, Chemosphere, 2005, vol. 58, pp. 595–604.
Girousse, C., Bournoville, R., and Bonnemain, J.L., Water deficit-induced changes in concentrations in proline and some other amino acids in the phloem sap of alfalfa, Plant Physiol., 1996, vol. 111, pp. 109–113.
Moussa, H. and Abdel-Aziz, S.M., Comparative response of drought tolerant and drought sensitive maize genotypes to water stress, Aust. J. Crop Sci., 2008, vol. 1, pp. 31–36.
Haghighi, S., Saki-Nejad, T., and Lack, S., Effect of biological fertilizer of humic acid on metabolic process of biological nitrogen fixation, Life Sci. J., 2011, vol. 8, pp. 43–48.
El-Nemr, M.A., El-Desuki, M., El-Bassiony, A.M., and Fawzy, Z.F., Response of growth and yield of cucumber plants (Cucumis sativus L.) to different foliar applications of humic acid and biostimulators, Aust. J. Basic Appl. Sci., 2012, vol. 6, pp. 630–637.
Cheng, F.J., Yang, D.Q., and Wu, Q.S., Physiological effects of humic acid on drought resistance of wheat, J. Appl. Ecol., 1995, vol. 6, pp. 363–367 [in Chinese].
Harbone, J.B., Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, London: Chapman and Hall, 1984.
Basu, P.S., Masood, A., and Chaturvedi, S.K., Adaptation of photosynthetic component of chickpea to water stress, Proc. 4th Int. Crop Sci. Congr. “New directions for a diverse planet” (26 September-1 October, 2004, Brisbane, Australia), Fischer, R.A., Ed., Brisbane Queensland: The Regular Institute Ltd., Aust. Soc. Agronomy, 2004.
Beers, R.F., Jr. and Sizer, I.W., A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase, J. Biol. Chem., 1952, vol. 195, pp. 133–140.
Hemeda, H.M. and Klein, B.P., Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts, J. Food Sci., 1990, vol. 55, pp. 184–185.
Nakano, Y. and Asada, K., Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts, Plant Cell Physiol., 1981, vol. 22, pp. 867–880.
Bates, L.S., Waldran, R.P., and Teare, I.D., Rapid determination of free proline for water studies, Plant Soil, 1973, vol. 39, pp. 205–208.
Bradford, M.N., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Cakmak, I. and Horst, J., Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max), Physiol. Plant., 1991, vol. 83, pp. 463–468.
Moran, J.F., Becana, M., Iturbe-Ormaetxe, I., Frechilla, S., Klucas, R.V., and Aparicio-Tejo, P., Drought induces oxidative stress in pea plants, Biol. Plant., 1994, vol. 194, pp. 346–352.
Rao, G.G. and Rao, G.R., Pigment composition and chlorophylase activity in pigeon pea (Cajanus indians Spreng.) and gingelly (Sesamum indicum L.) under NaCl salinity, Indian. J. Exp. Biol., 1981, vol. 19, pp. 768–770.
Singh, A.K. and Dubey, R.S., Changes in chlorophyll a and b contents and activities of photosystems I and II in rice seedlings induced by NaCl, Photosynthetica, 1995, vol. 31, pp. 489–499.
Liu, C., Cooper, R.J., and Bowman, D.C., Humic acid application affects photosynthesis, root development and nutrient of creeping bent grass, Hort. Sci., 1988, vol. 33, pp. 1023–1025.
Murkowski, A., Heat stress and spermidine effect on chlorophyll fluorescence in tomato plants, Biol. Plant., 2001, vol. 44, pp. 53–57.
Colom, M.R. and Vazzana, C., Photosynthesis and PSII functionality of drought-resistant and drought-sensitive weeping love grass plants, Environ. Exp. Bot., 2003, vol. 49, pp. 135–144.
Souza, R.P., Machadoa, E.C., Silva, J.A.B., Lagôa, A.M.M.A., and Silveira, J.A.G., Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery, Environ. Exp. Bot., 2004, vol. 51, pp. 45–56.
Gholami, H., Samavat, S., Oraghi, Ardebili, Z., The alleviating effects of humic substances on photosynthesis and yield of Plantago ovata in salinity conditions, Int. Res. J. Appl. Basic Sci., 2013, vol. 4, pp. 1683–1686.
Yamazaki, J., Ohashi, A., Hashimoto, Y., Negishi, E., Kumagai, S., Kubo, T., Oikawa, T., Maruta, E., and Kamimura, Y., Effects of high light and low temperature during harsh winter on needle photodamage of Abies mariesii growing at the forest limit on Mt. Norikura in Central Japan, Plant Sci., 2003, vol. 165, pp. 257–264.
Abedi, T. and Pakniyat, H., Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.), Czech. J. Genet. Plant Breed., 2010, vol. 46, pp. 27–34.
Chen, Y. and Aviad, T., Effects of humic substances on plant growth, Humic substances in soil and crop science, MacCarthy, P., Clapp, C.E., Malcolm, R.L., and Bloom, P.R., Eds., Madison: American Society of Agronomy and Soil Science Society of America, 1990, pp. 161–186.
Turkan, I., Bor, M., Ozdemir, F., and Koca, H., Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant Phaseolus acutifolius Gray and drought-sensitive Phaseolus vulgaris L. subjected to polyethylene glycol mediated water stress, Plant Sci., 2005, vol. 168, p. 223.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Lotfi, R., Gharavi-Kouchebagh, P. & Khoshvaghti, H. Biochemical and physiological responses of Brassica napus plants to humic acid under water stress. Russ J Plant Physiol 62, 480–486 (2015). https://doi.org/10.1134/S1021443715040123
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443715040123