Abstract
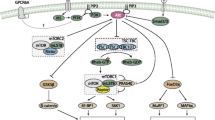
The mTOR enzyme belongs to the specific serine/threonine protein kinase family and plays an important role in extracellular signal transduction by phosphorylating many substrates in various metabolic pathways of the human body. The mTOR protein possessing protein kinase activity is encoded by the FRAP1 gene, which is located on chromosome 1 (1p36.2). In skeletal muscles, mTOR occurs as a component of two protein complexes, mTORC1 and mTORC2, which differ in sensitivity to the inhibitory effect of rapamycin. To regulate metabolism in skeletal muscles, mTOR phosphorylates various protein metabolism enzymes, transcription factors, and translation factors. Expression of mTOR is triggered in response to changes in metabolic demand of the muscle cell and intensifies protein metabolism. Studies of the past years showed that mTOR plays an important role in regulating intracellular metabolism, acting primarily at initiation and synthesis of muscle proteins. The review considers the current data on the role of mTOR in regulating the physiological function in skeletal muscles.
Similar content being viewed by others
References
Magnuson, B., Ekim, B., and Fingar, D., Regulation and function of ribosomal protein s6 kinase (S6K) with mTOR signaling networks, Biochem. J., 2012, vol. 441,part 1, p. 1.
Efeyen, A. and Sabatini, D.M., mTOR and cancer: Many loops in one pathway, Curr. Opin. Cell Biol., 2010, vol. 22, no. 2, p. 169.
Nader, C.A., Muscle growth learns new trick from an old dog, Nat. Med., 2007, vol. 13, no. 9, p. 1016.
Sancak, Y., Thoreen, C.C., Peterson, T.R., et al., PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase, Mol. Cell, 2007, vol. 25, no. 6, p. 903.
Vander Haar, E., Lee, S.I., Bandhakavi, S., et al., Insulin signaling to mTOR mediated by the Akt/PKB substrate PRAS40, Nat. Cell Biol., 2007, vol. 9, no. 3, p. 316.
Wang, H., Zhang, Q., Wen, Q., et al., Proline-rich Akt substrate of 40 kDa (PRAS40): A novel downstream target of PI3K/Akt signaling pathway, Cell Signal, 2012, vol. 24, no. 1, p. 17.
Zoncu, R., Efeyan, A., and Sabatini, D.M., mTOR: From growth signal integration to cancer, diabetes and ageing, Nat. Rev. Mol. Cell Biol., 2011, vol. 12, no. 1, p. 21.
Sarbassov, D.D., Guertin, D.A., Ali, S., et al., Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex, Science, 2005, vol. 307, no. 5751, p. 1098.
Demirkan, G., Yu, K., Boylan, J.M., et al., Phosphoproteomic profiling of in vivo signaling in liver by the mammalian target of rapamycin complex 1 (mTORC1), PLoS One, 2011, vol. 6, no. 6, p. e21729.
Hsu, P.P., Kang, S.A., Ramesender, J., et al., The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling, Science, 2011, vol. 332, no. 6034, p. 1317.
Laplante, M. and Sabatini, D.M., mTOR signaling in growth control and disease, Cell, 2012, vol. 149, no. 2, p. 274.
Johnson, S., Rabinovitch, P., and Kaeberlein, M., mTOR is a key modulator of ageing and age-related disease, Nature, 2013, vol. 493, no. 7432, p. 338.
Sengupta, S., Peterson, T.R., and Sabatini, D.M., Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress, Mol. Cell, 2010, vol. 40, no. 2, p. 310.
Huang, J. and Manning, B.D., The TSC1-TSC2 complex: A molecular switchboard controlling cell growth, Biochem. J., 2008, vol. 412, p. 179.
Soliman, G.A., Acosta-Jaquez, H.A., Dunlop, E.A., et al., Mtor Ser2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action, J. Biol. Chem., 2010, vol. 285, no. 11, p. 7866.
Ekim, B., Magnuson, B., Acosta-Jaquez, H.A., et al., mTOR kinase domain phosphorylation promotes mTORC1 signaling, cell growth and cell cycle progression, Mol. Cell Biol., 2011, vol. 31, no. 14, p. 2787.
Foster, K.G., Acosta-Jaquez, H.A., and Romeo, Y., Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation, J. Biol. Chem., 2010, vol. 285, no. 1, p. 80.
Carriere, A., Romeo, Y., Acosta-Jaquez, H.A., et al., ERK1/2 phosphorylate raptor to promote Ras-dependent activation of mTOR complex1 (mTORC1), J. Biol. Chem., 2011, vol. 286, no. 1, p. 567.
Goodman, C.A., Miu, M.H., Frey, J.W., et al., A phosphatidylinositol 3-kinase/protein kinase B-independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy, Mol. Biol. Cell, 2010, vol. 21, no. 19, p. 3258.
Sun, Y., Fang, Y., Yoon, M.S., et al., Phospholipase D1 is an effector of Rheb in the mTOR pathway, Proc. Natl. Acad. Sci. USA, 2008, vol. 105, no. 24, p. 8286.
Toschi, A., Lee, E., Xu, L., et al. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: Competition with rapamycin, Mol. Cell. Biol., 2009, vol. 29, no. 6, p. 1411.
Winter, J.N., Fox, T.E., Kester, M., et al., Phosphatidic acid mediates of mTORC1 through the ERK signaling pathway, Am. J. Physiol. Cell Physiol., 2010, vol. 299, no. 2, p. 335.
Yoon, M.S., Sun, Y., Arauz, E., et al., Phosphatidic acid activates mammalian target of rapamycin complex 1 (mTORC1) kinase by displacing FK506 binding protein 38 (FKBP38) and exerting an allosteric effect, J. Biol. Chem., 2011, vol. 286, no. 34, p. 29 568.
Kim, E. and Guan, K.L., Rac GTPases in nutrientmediated TOR signaling pathway, Cell Cycle, 2009, vol. 8, no. 7, p. 1014.
Kim, E., Goraksha-Hicks, P., Neufeld, T.P., et al., Regulation of TORC1 by Rag GTPases in nutrient response, Nat. Cell Biol, 2008, vol. 10, no. 8, p. 935.
Sancak, Y., Peterson, T.R., Shaul, Y.D., et al., The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1, Science, 2008, vol. 320, no. 5907, p. 1496.
Sancak, Y., Bar-Peled, L., Zoncu, R., et al., Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids, Cell, 2010, vol. 141, no. 2, p. 290.
Dodd, K.M. and Tee, A.R., Leucine and mTORC1-A complex relationship, Physiol. Endocrinol. Metab., 2012, vol. 302, no. 11, p. 1329.
Kume, K., Iizumi, Y., Shimada, M., et al., Role of N-end rule ubiquitin ligases UBP1 and UBP2 in regulating the leucine-mTOR signaling pathway, Genes. Cells, 2010, vol. 15, no. 4, p. 339.
Tasaki, T., Mulder, L.C., Iwamatsu, A., et al., Family of mammalian E3 ubiquitin ligases that contain the UBR box motif and recognize N-degrons, Mol. Cell Biol., 2005, vol. 25, no. 16, p. 7120.
Schieke, S.M., Phillips, D., and McCay, J., The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity, J. Biol. Chem., 2006, vol. 281, no. 37, p. 27643.
Cunningham, J.T., Rodges, J.T., Arlow, D.H., et al., mTOR controls mitochondrial oxidative function through a YY1-PGC-1a transcriptional complex, Nature, 2007, vol. 450, no. 7170, p. 736.
Hornberger, T.A., Stuppard, R., Conley, K.E., et al., Mechanical stimuli regulate rapamycin-sensitive signaling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism, Biochem. J., 2004, vol. 380,Pt. 3, p. 795.
Horenberger, T.A., Mechanotransduction are the regulation of mTORC1 signaling in skeletal muscle, Int. J. Biochem. Cell Biol., 2011, vol. 43, no. 8, p. 1267.
Hornberger, T.A., Chu, W.K., Mak, Y.W., et al., The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling, Proc. Natl. Acad. Sci. USA, 2006, vol. 103, no. 12, p. 4741.
O’Neil, T.K., Duffy, L.R., Frey, J.W., et al., The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions, J. Physiol., 2009, vol. 587, no. 2, p. 347.
You, J.S., Frey, J.W., and Hornberger, T.A., Mechanical stimulation induces mTOR signaling via an ERK-independent mechanism: Implications for a direct activation of mTOR by phosphatidic acid, PLoS ONE, 2012, vol. 7, p. e47258.
Deldieque, L., Atherton, P., Patel, R., et al., Decrease in Akt/PKB signaling in human skeletal muscle by resistance exercise, Eur. J. Appl. Physiol., 2008, vol. 104, no. 1, p. 57.
MacKenzie, M.G., Hamilton, D.L., Murray, J.T., et al., mVps34 is activated following high-resistance contractions, J. Physiol., 2009, vol. 587, no. 1, p. 253.
Bending, G., Grimmler, M., Huttner, I.G., et al., Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart, Gene Dev., 2006, vol. 20, no. 17, p. 2361.
De Acetus, M., Notte, A., Accorero, F., et al., Cardiac overexpression of melusin protects from dilated cardiomyopathy due to long-standing pressure overload, Circ. Res., 2005, vol. 96, no. 10, p. 1087.
Knoll, R. and Marston, S., On mechanosensation actomyosin interaction and hypertrophy, Trends Cardiovasc. Med., 2012, vol. 22, no. 1, p. 17.
Glass, D.J., PI3 kinase regulation of skeletal muscle hypertrophy and atrophy, Curr. Top. Microbiol. Immunol., 2010, vol. 346, no. 2, p. 267.
Yamada, A.K., Verienga, R., and Bueno Junior, C.R., Mechanotransduction pathways in skeletal muscle hypertrophy, J. Recept. Signal. Transduct. Res., 2012, vol. 32, no. 1, p. 42.
Philp, A., Hamilton, L., and Baar, K., Signaling mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1, J. Appl. Physiol., 2011, vol. 110, no. 2, p. 561.
Kravchenko, I.V., Furalyov, V.O., and Popov, V.O., Stimulation of mechanogrowth factor expression by myofibrillar proteins in murine myoblasts and myotubes, Mol. Cell Biochem., 2012, vol. 363, nos. 1–2, p. 347.
Deldieque, L., Atherton, P., Patel, R., et al., Decrease in Akt/PKB signaling in human skeletal muscle by resistance exercise, Eur. J. Appl. Physiol., 2008, vol. 108, no. 1, p. 57.
Fluck, M. and Hoppeler, H., Molecular basis of skeletal muscle plasticity—from gene to form and function, Rev. Physiol. Biochem. Pharmacol., 2003, vol. 146, p. 159.
Yang, Y., Creer, A., Jemiolo, B., et al., Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle, J. Appl. Physiol., 2005, vol. 98, no. 5, p. 1745.
Baar, K., Nader, G., and Bodine, S., Resistance exercise, muscle loading/unloading and the control of muscle mass, Essays Biochem., 2006, vol. 42, no. 1, p. 61.
Stepto, N.K., Coffey, V.G., Carey, A.L., et al., Global gene expression in skeletal muscle from well-trained strength and endurance athletes, Med. Sci. Sports Exerc., 2009, vol. 41, no. 3, p. 546.
Coffey, V.G., Shield, A., Canny, B.J., et al., Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes, Am. J. Physiol. Endocrinol. Metab., 2006, vol. 290, no. 4, p. 849.
Dreyer, H.C., Fujita, S., Cadenas, J.G., et al., Resistance exercise increases ANPK activity and reduces 4E-BP1 phosphorylation and protein syntheses in human skeletal muscle, J. Physiol., 2006, vol. 576, no. 2, p. 613.
Leger, B., Cartoni, R., Praz, M., et al., Akt signaling through GSK-3 β, mTOR and FOXO1 is involved in human skeletal muscle hypertrophy and atrophy, J. Physiol., 2006, vol. 576, no. 3, p. 923.
Wilkilson, S.B., Phillips, S.M., Atherton, P.J., et al., Differential effects of resistance and endurance exercise in the fed state on signaling molecule phosphorylation and protein synthesis in human muscle, J. Physiol., 2008, vol. 586, no. 15, p. 3701.
Coffey, V.G. and Hawley, J.A., The molecular bases of training adaptation, Sports Med., 2007, vol. 37, no. 9, p. 737.
Terzis, G., Georgiadis, G., Stratakos, G., et al., Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects, Eur. J. Appl. Physiol., 2008, vol. 102, no. 2, p. 145.
Churchley, E.G., Coffey, V.G., Pederson, D.J., et al., Influence of preexercise muscle glycogen content on transcriptional activity of metabolic and myogenic genes in well-trained humans, J. Appl. Physiol., 2007, vol. 102, no. 5, p. 1604.
Moore, D.A., Tang, J.E., Burd, N.A., et al., Differential stimulation of myofibrilar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise, J. Physiol., 2009, vol. 587, no. 4, p. 897.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.D. Golberg, A.M. Druzhevskaya, V.A. Rogozkin, I.I. Ahmetov, 2014, published in Fiziologiya Cheloveka, 2014, Vol. 40, No. 5, pp. 123–132.
Rights and permissions
About this article
Cite this article
Golberg, N.D., Druzhevskaya, A.M., Rogozkin, V.A. et al. Role of mTOR in the regulation of skeletal muscle metabolism. Hum Physiol 40, 580–588 (2014). https://doi.org/10.1134/S0362119714040070
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119714040070