Abstract
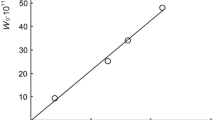
A reaction scheme is suggested for the initiated addition process. The scheme includes the reaction between the free 1: 1 adduct radical and the unsaturated reactant, which is in competition with chain propagation through a reactive free radical (\({}^ \cdot P{\mathbf{ }}Cl_2 ,(CH_3 )_2 \mathop C\limits^\cdot {\mathbf{ }}OH\), etc.) and yields a low-reactivity free radical (\(CH_2 = C(CH_3 )\mathop C\limits^\cdot {\mathbf{ }}H_2 ,{\mathbf{ }}CH_2 = CH\mathop C\limits^\cdot {\mathbf{ }}HOH\), etc.) inhibiting the nonbranched-chain process. Simple rate equations containing one to three parameters to be determined directly are set up using quasi-steady-state treatment. These equations provide good fits for the nonmonotonic (peaking) dependences of the formation rates of the molecular addition products (1: 1 adducts) on the concentration of the unsaturated component in liquid homogeneous binary systems consisting of a saturated component (PCl3, 2-propanol, etc.) and an unsaturated component (methylpropene, 2-propen-1-ol, etc.). The unsaturated compound in these systems is both a reactant and an autoinhibitor generating low-reactivity free radicals.
Similar content being viewed by others
References
Gurvich, L.V., Karachevtsev, G.V., Kondrat’ev, V.N., et al., Energii razryva khimicheskikh svyazei. Potentsialy ionizatsii i srodstvo k elektronu (Bond Dissociation Energies, Ionization Potentials, and Electron Affinity), Kondrat’ev, V.N., Ed., Moscow: Nauka, 1974.
Benson, S.W., Thermochemical Kinetics: Methods for the Estimation of Thermochemical Data and Rate Parameters, New York: Wiley, 1976, 2nd ed.
Pedley, J.B., Naylor, R.D., and Kirby, S.P., Thermochemical Data of Organic Compounds, London: Chapman & Hall, 1986, 2nd ed.
Orlov, Yu.D., Lebedev, Yu.A., and Saifullin, I.Sh., Termokhimiya organicheskikh svobodnykh radikalov (Thermochemistry of Organic Free Radicals), Kutepov, A.M., Ed., Moscow: Nauka, 2001.
Walling, Ch., Free Radicals in Solution, New York: Wiley, 1957.
Emanuel’, N.M., Denisov, E.T., and Maizus, Z.K., Tsepnye reaktsii okisleniya uglevodorodov v zhidkoi faze (Chain Oxidation Reactions of Hydrocarbons in the Liquid Phase), Moscow: Nauka, 1965.
Poluektov, V.A., Babkina, E.I., and Begishev, I.R., On the Dependence of the Rate of a Chain Reaction on the Reactant Ratio, Dokl. Akad. Nauk SSSR, 1974, vol. 215, no. 3, p. 649.
Silaev, M.M. and Bugaenko, L.T., Mathematical Simulation of the Kinetics of Radiation Induced Hydroxyalkylation of Aliphatic Saturated Alcohols, Radiat. Phys. Chem., 1992, vol. 40, no. 1, p. 1.
Silaev, M.M. and Bugaenko, L.T., Kinetics of the Addition of α-Hydroxyalkyl Radicals to 2-Propen-1-ol and Formaldehyde, Kinet. Katal., 1994, vol. 35, no. 4, p. 509.
Silaev, M.M., The Competition Kinetics of Radical-Chain Addition, Zh. Fiz. Khim., 1999, vol. 73, no. 7, p. 1180 [Russ. J. Phys. Chem. (Engl. Transl.), vol. 73, no. 7, p. 1050].
Bard, Y., Nonlinear Parameter Estimation, New York: Academic, 1974.
Urry, W.H., Stacey, F.W., Huyser, E.S., and Juveland, O.O., The Peroxide-and Light-Induced Additions of Alcohols to Olefins, J. Am. Chem. Soc., 1954, vol. 76, no. 2, p. 450.
Urry, W.H. and Juveland, O.O., Free Radical Additions of Amines to Olefins, J. Am. Chem. Soc., 1958, vol. 80, no. 13, p. 3322.
Shostenko, A.G., Zagorets, P.A., Dodonov, A.M., and Greysh, A.A., γ-Radiation-Induced Addition of Phosphorus Trichloride to Isobutylene, Khim. Vys. Energ., 1970, vol. 4, no. 4, p. 357.
Kim, V., Shostenko, A.G., and Gasparyan, M.D., Reactivity of Polychloroalkyl Radicals in the Telomerization of CCl4 with 1-Propene and 2-Methyl-1-Propene (in Russian), React. Kinet. Catal. Lett., 1979, vol. 12, no. 4, p. 479.
Myshkin, V.E., Shostenko, A.G., Zagorets, G.A., et al., Determination of Absolute Rate Constants for the Addition of the Ethyl Radical to Olefins, Teor. Eksp. Khim., 1977, vol. 13, no. 2, p. 266.
Zamyslov, R.A., Shostenko, A.G., Dobrov, I.V., and Tarasova, N.P., Kinetics of γ-Radiation-Induced Reactions of 2-Propanol with Trifluoropropene and Hexafluoropropene, Kinet. Katal., 1987, vol. 28, no. 4, p. 977.
Silaev, M.M., Dependence of Radiation-chemical γ-Diol Yields on the 2-Propen-1-ol Concentration in the Radiolysis of Aliphatic Saturated C1-C3 Alcohol + 2-Propen-1-ol Systems, Khim. Vys. Energ., 1990, vol. 24, no. 3, p. 282.
Afanas’ev, A.M., Bugaenko, L.T., Kalyazin, E.P., et al., USSR Inventor’s Certificate no. 805 599, Byull. Izobret., 1982, no. 3.
Silaev, M.M., γ-Diol Formation via the Autooxidation of 2-Propen-1-ol Solutions in Saturated Alcohols, Vestn. Mosk. Univ., Ser. 2: Khim., 1994, vol. 35, no. 1, p. 40.
Silaev, M.M., RF Patent 2 030 382, Byull. Izobret., 1995, no. 7.
Bugaenko, L.T., Kuz’min, M.G., and Polak, L.S., Khimiya vysokikh energii (High-Energy Chemistry), Polak, L.S., Ed., Moscow: Khimiya, 1988, p. 99.
Thomas, J.K., Pulse Radiolysis of Aqueous Solutions of Methyl Iodide and Methyl Bromide. The Reactions of Iodine Atoms and Methyl Radicals in Water, J. Phys. Chem., 1967, vol. 71, no. 6, p. 1919.
Vereshchinskii, I.V. and Pikaev, A.K., Vvedenie v radiatsionnuyu khimiyu (Introduction to Radiation Chemistry), Spitsyn, V.I., Ed., Moscow: Akad. Nauk SSSR, 1963.
Author information
Authors and Affiliations
Additional information
Original Russian Text © M.M. Silaev, 2007, published in Teoreticheskie Osnovy Khimicheskoi Tekhnologii, 2007, Vol. 41, No. 3, pp. 289–295.
Rights and permissions
About this article
Cite this article
Silaev, M.M. Simulation of the nonbranched-chain addition of saturated free radicals to alkenes and their derivatives yielding 1: 1 adducts. Theor Found Chem Eng 41, 273–278 (2007). https://doi.org/10.1134/S0040579507030062
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0040579507030062