Abstract
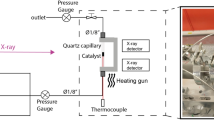
The MgSnO3-450 and MgSnO3-750 catalysts of different morphologies, synthesized by calcining magnesium hydroxystannate MgSn(OH)6 at 450 and 750°C, respectively, are studied in the aldol condensation of acetone under supercritical conditions (400°C and a pressure of 12.0 MPa). The structure of the catalysts is characterized via XRD, SEM, TEM, DSC, and BET. The X-ray amorphous MgSnO3-450 sample exhibits high activity (0.42 mol/(h gcat)) and selectivity to isomeric phorones (40%). The MgSnO3-750 catalyst, which has a higher degree of crystallinity, exhibits lower activity (0.33 mol/(h gcat)) and a high selectivity to isomeric mesityl oxides (up to 70%). Comparing the data on the phase composition of the catalysts and the results of the reaction, it can be supposed that the phase responsible for the selective condensation of two acetone molecules into mesityl oxide on the MgSnO3-750 catalyst is magnesium orthostannate Mg2SnO4. The formation of a MgSnO3 magnesium metastannate phase in the X-ray amorphous MgSnO3-450 catalyst contributes to the formation of phorones, which are products of the condensation of three acetone molecules. It is found that the structure of the MgSnO3-450 catalyst is rearranged during the acetone condensation under supercritical conditions.








Similar content being viewed by others
REFERENCES
Y. Ono, J. Catal. 216, 406 (2003). https://doi.org/10.1016/S0021-9517(02)00120-3
H. Hattori, Appl. Catal. A.: Gen. 222, 247 (2001). https://doi.org/10.1016/S0926-860X(01)00839-0
A. E. Koklin, G. M. Khasyanova, L. M. Glukhov, and V. I. Bogdan, Russ. Chem. Bull. 66, 488 (2017).https://doi.org/10.1007/s11172-017-1760-5
G. A. Veshchitsky, A. V. Smirnov, N. V. Mashchenko, et al., Russ. J. Phys. Chem. B 15, 1299 (2021). https://doi.org/10.1134/S1990793121080133
E. S. Alekseev, A. Yu. Alentiev, A. S. Belova, et al., Russ. Chem. Rev. 89, 1337 (2020). https://doi.org/10.1070/RCR4932
Z. Lu, J. Liu, Y. Tang, and Y. Li, Inorg. Chem. Commun. 7, 731 (2004). https://doi.org/10.1016/j.inoche.2004.03.030
F. Huang, Z. Yuan, H. Zhan, et al., Mater. Chem. Phys. 83, 16 (2004). https://doi.org/10.1016/j.matchemphys.2003.07.016
A. I. Aparnev, L. I. Afonina, A. V. Loginov, and N. F. Uvarov, Russ. J. Appl. Chem. 89, 212 (2016). https://doi.org/10.1134/S1070427216020087
G. Pfaff, Thermochim. Acta 237, 83 (1994). https://doi.org/10.1016/0040-6031(94)85186-7
A. Taherkhani and A. T. Asghari, Adv. Mater. Res. 403, 640 (2011). https://doi.org/10.4028/www.scientific.net/amr.403-408.640
M. M. Rashad and H. El-Shall, Powder Technol. 183, 161 (2008). https://doi.org/10.1016/j.powtec.2007.07.019
K. Fujiwara, H. Minato, J. Shiogai, et al., APL Mater. 7, 022505 (2019). https://doi.org/10.1063/1.5054289
ICDD, The Powder Diffraction File (Int. Centre for Diffraction Data, Newtown Square, PA, 2018).
T. V. Bogdan, A. E. Koklin, I. V. Mishin, et al., Russ. Chem. Bull. 71, in press (2022).
ACKNOWLEDGMENTS
The authors thank S.V. Savilov and S.V. Maksimov (Department of Chemistry, Lomonosov Moscow State University (MSU)) for TG–DSC and TEM carried out at “Nanochemistry and Nanomaterials” MSU Equipment Center acting under Lomonosov Moscow State University Program of Development. The authors are grateful to the Department of Structural Studies of the Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences (IOC RAS) for performing SEM studies of the samples; and to the Center for Advanced Catalytic Technologies of the IOC RAS for studying the textural characteristics of our samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare they have no conflict of interest.
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Bogdan, T.V., Koklin, A.E., Mashchenko, N.V. et al. Structure and Catalytic Properties of Magnesium Stannate in the Acetone Condensation Reaction. Russ. J. Phys. Chem. 96, 2344–2352 (2022). https://doi.org/10.1134/S0036024422110048
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422110048