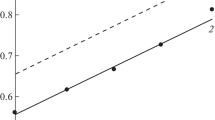
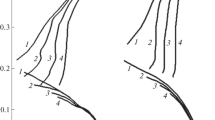
Abstract
Potentiometry and voltammetry with a rotating disk electrode are used to study the corrosion of St3 low-carbon steel in 1 M HCl containing dissolved molecular oxygen from the mass loss of metal samples in a static and dynamic aggressive environment. It is shown that molecular oxygen in the acid solution and the transition from the static to dynamic state of an aggressive medium accelerates the corrosion of steel. The corrosion of steel in this environment includes the anodic ionization of steel in the kinetic region and two partial cathodic reactions: the evolution of hydrogen and the reduction of dissolved molecular oxygen, characterized by kinetic and diffusion controls, respectively. Modeling the effect the hydrodynamic mode of the motion of a corrosive medium has on the rate of the cathodic reduction of molecular O2 on low-carbon steel using the Levich equation and comparing the results to experimental data suggests with high probability that in the flow of a corrosive medium it mainly proceeds according to the scheme O2 + 2H+ + 2e = H2O2. The values of the true kinetic currents of the cathodic reaction are estimated for a steel disk electrode in 1 M HCl that is freely aerated with air and forcibly aerated with gaseous O2. The effective coefficient of diffusion of dissolved molecular O2 in 1 M HCl is established.






Similar content being viewed by others
REFERENCES
H. Kaesche, Die Korrosion der Metalle. Physikalisch-chemische Prinzipien und aktuelle Probleme (Springer, Berlin, 1979).
L. I. Antropov, Theoretical Electrochemistry (Vyssh. Shkola, Moscow, 1965), p. 348 [in Russian].
J. O. Bockris, D. Drazic, and A. R. Despic, Electrochim. Acta 4, 325 (1961). https://doi.org/10.1016/0013-4686(61)80026-1
R. J. Chin and K. Nobe, J. Electrochem. Soc. 119, 1457 (1972). https://doi.org/10.1149/1.2404023
F. Si, Y. Zhang, L. Yan, et al., Rotating Electrode Methods and Oxygen Reduction Electrocatalysts, Ed. by W. Xing, G. Yin, and J. Zhang (Elsevier, Amsterdam, 2014), p. 133. https://doi.org/10.1016/B978-0-444-63278-4.00004-5
V. M. Andoralov, M. R. Tarasevich, and O. V. Tripachev, Russ. J. Electrochem. 47, 1327 (2011). https://doi.org/10.1134/S1023193511120020
Z. Zhao and P. K. Shen, Electrochemical Oxygen Reduction, Ed. by P. K. Shen (Springer, Singapore, 2021), p. 11. https://doi.org/10.1007/978-981-33-6077-8_2
L. Vracar, Encyclopedia of Applied Electrochemistry, Ed. by G. Kreysa, K. Ota, and R. F. Savinell (Springer Science, New York, 2014), p. 1485.
Z. Jia, G. Yin, and J. Zhang, in Rotating Electrode Methods and Oxygen Reduction Electrocatalysts, Ed. by W. Xing, J. Zhang, and G. Yin (Elsevier, Amsterdam, 2014), p. 199. https://doi.org/10.1016/B978-0-444-63278-4.00006-9
Ya. G. Avdeev, K. L. Anfilov, and Yu. I. Kuznetsov, Int. J. Corros. Scale Inhib. 10, 1566 (2021). https://doi.org/10.17675/2305-6894-2021-10-4-12
Yu. V. Pleskov and V. Yu. Filinovskii, The Rotating Disk Electrode (Consultants Bureau, New York, 1976).
I. Dumitrescu and R. M. Crooks, Proc. Natl. Acad. Sci. U. S. A. 109, 11493 (2012). https://doi.org/10.1073/pnas.1201370109
C. Du, Q. Tan, G. Yin, and J. Zhang, in Rotating Electrode Methods and Oxygen Reduction Electrocatalysts, Ed. by W. Xing, G. Yin, and J. Zhang (Elsevier, Amsterdam, 2014), p. 171. https://doi.org/10.1016/B978-0-444-63278-4.00005-7
A. L. Colley, J. V. Macpherson, and P. R. Unwin, Electrochem. Commun. 10, 1334 (2008). https://doi.org/10.1016/j.elecom.2008.06.032
Short Handbook of Physical Chemical Values, Ed. by K. P. Mishchenko and A. A. Ravdel’ (Khimiya, Leningrad, 1967), p. 103 [in Russian].
W. Xing, M. Yin, Q. Lv, et al., in Rotating Electrode Methods and Oxygen Reduction Electrocatalysts, Ed. by W. Xing, J. Zhang, and G. Yin (Elsevier, Amsterdam, 2014), p. 1. https://doi.org/10.1016/B978-0-444-63278-4.00001-X
N. A. Mel’nichenko, Russ. J. Phys. Chem. A 82, 1533 (2008). https://doi.org/10.1134/S0036024408090239
D. Tromans, Hydrometallurgy 50, 279 (1998). https://doi.org/10.1016/S0304-386X(98)00060-7
G. W. Hung and R. H. Dinius, J. Chem. Eng. Data 17, 449 (1972). https://doi.org/10.1021/je60055a001
A. N. Frumkin and E. A. Aikazyan, Dokl. Akad. Nauk SSSR 100, 315 (1955).
A. N. Frumkin and G. A. Tedoradze, Dokl. Akad. Nauk SSSR 118, 530 (1958).
X. Wang, Z. Li, Y. Qu, et al., Chemistry 5, 1486 (2019). https://doi.org/10.1016/j.chempr.2019.03.002
W. N. Wermink and G. F. Versteeg, Ind. Eng. Chem. Res. 56, 3775 (2017). https://doi.org/10.1021/acs.iecr.6b04606
W. N. Wermink and G. F. Versteeg, Ind. Eng. Chem. Res. 56, 3789 (2017). https://doi.org/10.1021/acs.iecr.6b04641
Ya. G. Avdeev and T. E. Andreeva, Russ. J. Phys. Chem. A 95, 1128 (2021). https://doi.org/10.1134/S0036024421060029
Ya. G. Avdeev and T. E. Andreeva, Russ. J. Phys. Chem. A 96, 425 (2022). https://doi.org/10.1134/S0036024422020030
Funding
This work was supported by R&D 2022–2024 “Chemical Resistance of Materials: Protecting Metals and Other Materials from Corrosion and Oxidation,” EGISU registration no. 122011300078-1, inventory number FFZS-2022-0013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Drozdova
Rights and permissions
About this article
Cite this article
Avdeev, Y.G., Andreeva, T.E. Mechanism of Corrosion of Low-Carbon Steel in 1 M Solutions of Hydrochloric Acid Saturated with Oxygen. Russ. J. Phys. Chem. 96, 2189–2197 (2022). https://doi.org/10.1134/S0036024422100041
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422100041