Abstract
A study is performed of the electrocatalytic properties of 2,2'-bipyridine in the presence of acids of different natures (HBF4, HClO4, TsOH, CF3COOH) and the nature of the synergistic effect. It is shown that the pKa of the acids greatly affect the mechanism of the reactions that occur. Based on electrochemical and electrocatalytic data obtained via cyclic voltammetry and preparative potentiostatic electrolysis, main mechanisms of the electrocatalytic production of molecular hydrogen are proposed that depend on the nature of the acids. The thermodynamic and kinetic parameters of the considered systems are calculated.
Similar content being viewed by others
INTRODUCTION
In recent decades, hydrogen has been actively considered as an alternative fuel with a number of advantages over traditional energy sources [1–3]. Developments in the field of creating highly efficient and relatively inexpensive large-scale catalytic systems for the production of molecular hydrogen can rightly be considered a cornerstone of modern energy [4]. A great many transition metal complexes with fairly high catalytic activity have now been developed, but is of greater interest to create unique systems that do not use expensive metals [5–7]. An example is polyfluorinated porphyrin complexes, with which the potential of reduction is comparable to that of metal complex analogs [8]. The number of such systems is nevertheless small, and the question of creating and studying them in detail remains open.
It is known that introducing a basic atom into the composition of a catalyst considerably raises the electrocatalytic activity in reactions of molecular hydrogen generation [9]. A great many studies of the electrocatalytic properties of pyridine and other organic systems were therefore performed [10]. The promising results from these works show that organic systems of this type have huge potential in electrocatalytic processes for the production of molecular hydrogen.
The authors of [11–13] proposed metalless electrocatalysts that differed in a number of characteristics (stability, availability, environmental friendliness) from all known catalysts based on transition metals. In a continuation of the ideas described above, this work considers the electrocatalytic properties of 2,2'-bipyridine:

It is assumed that the combination of two pyridine fragments connected to each other in the ortho-position contribute to the emergence of a synergistic effect that radically changes the mechanism of hydrogen generation from bimolecular elimination to a monomolecular reaction. This lifts the kinetic limitation imposed on the bimolecular stage, which is the limit stage in the reaction of obtaining molecular hydrogen in the presence of pyridine derivatives.
EXPERIMENTAL
All electrochemical measurements were made in a special three-electrode cell (20 mL) using a GAMRY REFERENCE 3000 digital potentiostat–galvanostat (Canada) connected to a personal computer. A glassy carbon electrode was used as the working electrode (S = 0.0314 cm2). The auxiliary electrode was platinum, and the reference electrode was standard silver chloride (E0 = 0.33 V (CH3CN) vs. Fc/Fc+). The background electrolyte was A 0.1 M solution of (n‑C4H9)4N)BF4. Cyclic voltammetry (CV) was used to study the mechanism and kinetics of the electrocatalytic reaction for the production of molecular hydrogen in the presence of bipyridines. The efficiency of the catalytic process and the stability of the catalytic systems were estimated via precursory potentiostatic electrolysis.
The concentration of catalyst was in all cases 1 × 10−3 mol/L. A ×100 excess of acid during electrolysis was selected.
The working electrode was cleaned with acetone after each measurement, and the three-electrode cell was washed with distilled water and acetone. The measurements were made at room temperature. All solutions were preliminarily deaerated with argon.
RESULTS AND DISCUSSION
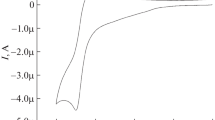
Cyclic voltammetry (CV) was used to study the electrochemical characteristics of 2,2'-bipyridine. As can be seen from the voltammogram (Fig. 1), the compound was redox active in the cathodic region, and no electrochemical activity was observed in the anodic region. Note the formation of two single-electron irreversible waves at potentials of −1.65 and −1.95 V. This indicates the successive reduction of each pyridine ring with the formation of temporally unstable anion radicals.
The properties of 2,2'-bipyridine were studied using acids of different natures (HBF4, HClO4, TsOH, CF3COOH) as sources of H+. Note that adding solutions of strong acids (pKa(CH3CN) HBF4) = 1.8; pKa(CH3CN) HClO4) = 2.1) greatly altered the initial CVA curve (Fig. 2).
In both cases, the initial waves shifted to the anode region \(E_{p}^{c}\) = −0.56 and −0.58 V, respectively. In addition, stepwise additions of acid produced a linear increase in current until the second peak was completely smoothed.
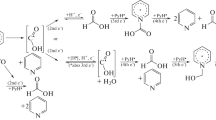
The identical potential values of the resulting peaks show that the considered processes were the same. The below mechanism of the electrocatalytic reaction for the production of molecular hydrogen may therefore be considered possible:

Protonation of the radicals formed during the reduction of one of the pyridine rings of the radical probably occurs because of its nitrogen atom, resulting in the formation of radical cations. The subsequent reduction of the intermediate promotes the electrocatalytic reaction. The presented mechanism fully reflects the possibility of a synergistic effect–in this case, the proposed substitution of bimolecular elimination for monomolecular stepwise reduction of hydrogen. This is possible when there are no steric hindrances in rotation around the C–C bond between pyridine fragments, side processes and chemical transformation of the intermediate are impossible, and the energy barrier of the process is apparently lowered.
A completely different form of the CVA curve is observed when using such weak acids as P-toluenesulfonic (pKa (CH3CN) = 8.5) and trifluoroacetic (рKa (CH3CN) = 10.6), so a different path of the process is assumed (Fig. 3).
The initial peaks shift to the anodic region upon adding P-toluenesulfonic acid (\(E_{p}^{c}\) = −0.70 and ‒0.82 V, respectively). Note that raising the concentration of acid in the solution increases the current in the first peak and transforms the second one considerably. The peak current reaches a plateau at a 2 mM concentration of acid. Later, however, we can see a rise in the current at a potential of −1.1 V. As expected, we can also see a shift of the initial waves to the anode region when using trifluoroacetic acid. At first glance, we cannot immediately judge the uniformity of the ongoing processes, but (as noted in the voltammogram) the values of the peak potentials coincide with those obtained using TsOH (\(E_{p}^{c}\) = −0.71 and −1.1 V, respectively). The accumulation of an intermediate compound (the formation of another intermediate) is most likely observed during the electrocatalytic reaction when using P-toluenesulfonic acid instead of trifluoroacetic acid.
The proposed mechanism is in this case described by the disproportionation of the cation formed as a result of the stepwise reduction to a biradical with subsequent elimination of molecular hydrogen. The higher energy barrier of the process shifts the waves to a more cathodic region than when strong acids are used.
Adding acids to an acetonitrile solution of 2,2'-bipyridine thus results in an electrocatalytic reaction of hydrogen production. However, the mechanism of the processes varies, depending on the nature of the acid that is used. It is quite surprising that the pKa of the acid does not seriously affect the strength of the current (Fig. 4).
Precursory potentiostatic electrolysis was performed at the potentials of the first half-waves [14]. Our results are shown in Table 1. This allowed us to understand whether the considered systems were stable and determine the efficiency of the ongoing electrocatalytic processes. The data in Table 1 confirm the electrocatalytic activity of 2,2'-bipyridine in the presence of all of our acids, since reduction was not observed when there was no catalyst. The greatest activity was seen for HBF4 and gradually fell in the order HClO4–TsOH–CF3COOH. Nevertheless, these experimental data are by no means satisfactory. The rapid loss of catalytic activity is most likely associated with side chemical processes. One way of solving this problem could be to introduce bulky acceptor substituents in the ortho- and para-positions of 2,2'-bipyridine to shield reactive intermediates.
CONCLUSIONS
Metal-free electrocatalytic systems based on 2,2'-bipyridine and acids of different natures (HBF4, HClO4, TsOH, CF3COOH) were studied and characterized. The main parameters of the ongoing processes were considered; possible intermediate compounds, thermodynamic and kinetic characteristics of the studied systems were determined; and the effect acids have on catalytic activity was examined. Main mechanisms of electrocatalytic reduction of molecular hydrogen were proposed on the basis of our data, and the possibility of a synergistic effect was confirmed. The results from this work could be useful in further studies of electrocatalytic systems based on organic heterocyclic compounds (especially bipyridines, which can be modified to increase the efficiency of the process).
REFERENCES
J. A. Turner, Science (Washington, DC, U. S.) 305, 972 (2004). https://doi.org/10.1126/science.1103197
S. A. Sherif, F. Barbir, and T. N. Veziroglu, Sol. Energy 78, 647 (2005). https://doi.org/10.1016/j.solener.2005.01.002
I. Dincer, Int. J. Hydrogen Energy 37, 1954 (2012). https://doi.org/10.1016/j.ijhydene.2011.03.173
Y. Zheng, Y. Jiao, Y. Zhu, et al., Nat. Commun. 5, 1 (2014). https://doi.org/10.1038/ncomms4783
O. Pantani, E. Anxolabehere-Mallart, A. Aukauloo, et al., Electrochem. Commun. 9, 54 (2007). https://doi.org/10.1016/j.elecom.2006.08.036
J. P. Cao, T. Fang, L. Z. Fu, et al., Int. J. Hydrogen Energy 39, 10980 (2014). https://doi.org/10.1016/j.ijhydene.2014.05.082
C. Tang, R. Zhang, W. Lu, et al., Angew. Chem. Int. Ed. 37, 1127 (2016). https://doi.org/10.1002/anie.201608899
Y. Wu, N. Rodríguez-López, and D. Villagrán, Chem. Sci. 9, 4689 (2018). https://doi.org/10.1039/c8sc00093j
V. Artero and M. Fontecave, Coord. Chem. Rev. 249, 1518 (2005). https://doi.org/10.1016/j.ccr.2005.01.014
S. G. Mairanovskii, Russ. Chem. Rev. 33, 118 (1964). https://doi.org/10.1016/S0022-0728(63)80149-7
A. V. Dolganov, B. S. Tanaseichuk, D. N. Moiseeva, et al., Electrochem. Commun. 68, 59 (2016). https://doi.org/10.1016/j.elecom.2016.04.015
A. V. Dolganov, B. S. Tanaseichuk, V. Y. Yurova, et al., Int. J. Hydrogen Energy 44, 21495 (2019). https://doi.org/10.1016/j.ijhydene.2019.06.067
A. V. Dolganov, B. S. Tanaseichuk, M. K. Pryanichnikova, et al., J. Phys. Org. Chem. 32, 3930 (2019). https://doi.org/10.1002/poc.3930
J. A. S. Roberts and R. M. Bullock, Inorg. Chem. 52, 3823 (2013). https://doi.org/10.1021/ic302461q
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 19-33-90094.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare they have no conflicts of interest.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ganz, O.Y., Klimaeva, L.A., Chugunov, D.B. et al. Synergetic Effect during the Electrocatalytic Reaction of Hydrogen Production in the Presence of 2.2'-Bipyridine. Russ. J. Phys. Chem. 96, 954–957 (2022). https://doi.org/10.1134/S0036024422050120
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422050120