Abstract
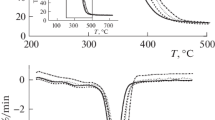
The thermal decomposition of polyethylene glycol fumarate–acrylic acid copolymer is investigated at different rates of heating. It is shown that increasing the rate of heating raises the temperature of the onset of decomposition. The kinetic parameters of decomposition are calculated using the integral Kissinger–Akahira–Sunose procedure. It is found that at different degrees of conversion, the activation energies are very close: E = 205–227 kJ/mol. The effect the composition of the copolymer has on the results from kinetic calculations is shown. The Coates–Redfern approach is used to determine the pre-exponential factor and the model of thermal decomposition. Calculated thermogravimetric curves are constructed and compared to experimental ones.





Similar content being viewed by others
REFERENCES
H. V. Boenig, Unsaturated Polyesters: Structure and Properties (Elsevier, Amsterdam, 1964).
C. Benny and T. T. Eby, J. Appl. Polym. Sci. 100, 457 (2006). https://doi.org/10.1007/s11595-009-4627-2
S. V. Vinogradova, V. V. Korshak, V. I. Kul’chitskii, et al., Polymer Sci. U. S. S. R. 10, 1757 (1968). https://doi.org/10.1016/0032-3950(68)90368-7
K. G. Johnson and L. S. Yang, in Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters, Ed. by J. Scheirs and T. E. Long (Wiley, West Sussex, UK, 2004), p. 697.
E. Kicko-Walczak, J. Appl. Polym. Sci. 88, 2851 (2003). https://doi.org/10.1002/app.11723
M. Zh. Burkeev, A. Z. Sarsenbekova, Y. M. Tazhbayev, and I. V. Figurinene, Russ. J. Phys. Chem. A 89, 2183 (2015). https://doi.org/10.1134/S0036024415120067
A. Z. Sarsenbekova, G. K. Kudaibergen, M. Zh. Burkeev, and G. K. Burkeeva, Russ. J. Phys. Chem. A 93, 1252 (2019). https://doi.org/10.1134/S0044453719060281
S. A. Vyazovkin, K. Burnham, J. M. Criadoc, et al., Thermochim. Acta 520, 1 (2011). https://doi.org/10.1016/j.tca.2011.03.034
S. Vyazovkin, K. Chrissafis, M. L. di Lorenzo, et al., Thermochim. Acta 590, 1 (2014). https://doi.org/10.1016/j.tca.2014.05.036
M. Zh. Burkeev, G. K. Kudaibergen, G. K. Burkeeva, T. M. Seilkhanov, E. M. Tazhbaev, J. Hranicek, A. V. Omasheva, and S. Zh. Davrenbekov Russ. J. Appl. Chem. A 91, 1145 (2018). https://doi.org/10.1134/S1070427218070121
H. E. Kissinger, Anal. Chem. 29, 1702 (1957). https://doi.org/10.1021/ac60131a045
T. Akahira and T. Sunose, Sci. Technol. 16, 22 (1971). https://doi.org/10.17221/115/2016-RAE
A. W. Coats and J. P. Redfern, Nature (London, U.K.) 201, 68 (1964). https://doi.org/10.1038/201068a0
A. A. Koptelov, I. A. Koptelov, A. A. Rogozina, and E. S. Yushkov, Russ. J. Appl. Chem. 89, 1454 (2016). https://doi.org/10.1134/S1070427216090111
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burkeev, M.Z., Bolatbay, A.N., Sarsenbekova, A.Z. et al. Integral Ways of Calculating the Destruction of Copolymers of Polyethylene Glycol Fumarate with Acrylic Acid. Russ. J. Phys. Chem. 95, 2009–2013 (2021). https://doi.org/10.1134/S0036024421100034
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421100034