Abstract
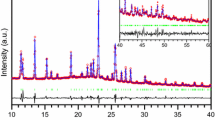
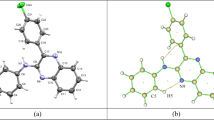
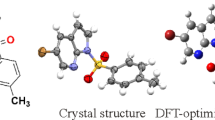
5-Azidomethyl-8-hydroxyquinoline has been synthesized and characterized using IR, 1H and 13C NMR spectroscopic methods. Thermal analysis revealed no solid-solid phase transitions. The crystal structure of this compound was refined by Rietveld method from powder X-ray diffraction data at 295 K. The single- crystal structure of the compound at 260 K was solved and refined using SHELX 97 program. According to the data obtained by both methods, the structure of the compound is monoclinic, space group P21/c, with Z = 4 and Z' = 1. For the single crystal at 260 K, a = 12.2879 (9) Å, b = 4.8782 (3) Å, c = 15.7423 (12) Å, β=100.807(14)°. Mechanisms of deformation resulting from intra- and intermolecular interactions, such as hydrogen bonding, induced slight torsions in the crystal structure. The optimized molecular geometry of 5-azidomethyl-8-hydroxyquinoline in the ground state is calculated using density functional theory (B3LYP) and Hartree-Fock (HF) methods with the 6-311G(d,p) basis set. The calculated results show good agreement with experimental values. Energy gap of the molecule was found using HOMO and LUMO calculation which reveals that charge transfer occurs within the molecule.
Similar content being viewed by others
References
M. di Vaira, C. Bazzicalupi, P. Orioli, L. Messori, B. Bruni, and P. Zatta, Inorg. Chem. 43, 3795 (2004).
A. Mellah and D. Benachour, Hydrometallurgy. 81, 100 (2006).
A. Albert and J. N. Phillips, Chem. Soc. 264, 1294 (1956).
R. Kayyali, A. S. Pannala, H. Khodr, and R. C. Hider, Biochem Pharmacol. 55, 1327 (1998).
I. Cacciatore, E. Fornasari, L. Baldassare, C. Cornacchia, S. Fulle, E. S. di Filippo, T. Pietrangelo, and F. Pinnen, Pharm. 6, 54 (2013).
Y. Hamada, IEEE Trans. Electron Devices. 44, 1208 (1997).
C. H. Chen and Shi Jianmin, J. Coord. Chem. Rev. 171, 161 (1998).
D. H. Mathew and H. B. Schlegel, Chem. Mater. 13, 2632 (2001).
V. A. Montes, R. Pohl, J. Shinar, and P. Anzenbacher, Jr., Chem. Eur. J. 12, 4523 (2006).
G. N. Lipunovaab, E. V. Nosovaab, V. N. Charushinab, and O. N. Chupakhinab, Comm. Inorg. Chem. 34, 142 (2014).
G. E. Collis, A. K. Burrell, K. D. John, and P. G. Plieger, Acta Crystallogr. C 59, 0443 (2003).
C. H. Chen and J. M. Shi, Coord. Chem. Rev. 171, 161 (1998).
A. Y. Shen, S. N. Wu, and C. T. Chiu, J. Pharm. Pharmacol. 51, 543 (1999).
D. Mechlovich, T. Amit, S. A. Mandel, O. Bar-Am, K. Bloch, M. B. H. Vardi, and P. Youdim, J. Pharmacol. Exp. Ther. 333, 874 (2010).
C. I. Nieto, M. A. Garcia, M. A. Farran, R. M. Claramunt, M. C. Torralba, M. R. Torres, I. Alkorta, and J. Elguero, J. Mol. Struct. 1008, 88 (2012).
R. Desiderato, J. C. Jerry, and G. R. Freemant, Acta Crystallogr. B 27, 2443 (1971).
D. E. Pearson, R. D. Wysong, and C. V. Breder, J. Org. Chem. 32, 2358 (1967).
H. Gershon, M. W. McNeil, and A. T. Grefig, J. Org. Chem. 34, 3268 (1969).
K. Hunger, Industrial Dyes, Chemistry, Properties, Applications (Wiley-VCH, Weinheim, 2003).
M. La Deda, A. Grisolla, I. Aiello, A. Crispini, M. Ghedini, S. Belviso, M. Amati, and F. Lelj, J. Chem. Soc., Dalton Trans. 16, 2424 (2004).
A. Saylam, Z. Seferoglu, and N. Ertan, J. Mol. Liq. 195, 267 (2014).
B. Himmi, B. Messnaoui, S. Kitane, A. Eddaif, A. Alaoui, A. Bouklouz, and M. Soufiaoui, Hydrometallurgy 93, 39 (2008).
M. Labrador, M. A. Cuevas-Diarte, D. Mondieig, and Y. Haget, Thermochim. Acta 195, 261 (1992).
H. M. Rietveld, J. A. Crystallog. 2, 65 (1969).
G. Sheldrick, SHELXS-97 Program for the Refinement of Crystal Structure (Univ. Göttingen, Göttingen, Germany, 1997).
A. El Assyry, B. Benali, A. Boucetta, and B. Lakhrissi, J. Mater. Environ. Sci. 5, 1860 (2014).
Y. Li, Y. Y. Liu, X. J. Chen, X. H. Xiong, and F. S. Li, PLoS ONE 9, e91361 (2014).
M. J. Frisch et al., Gaussian 03, Revision D.01 and D.02 (Gaussian Inc., Wallingford, CT, 2005).
A. Kadiri, B. Kabouchi, B. Benali, C. Cazeau-Dubroca, and G. Nouchi, Spectrochim. Acta, Part A 50, 1 (1994).
C. Cazeau-Dubroca, Trends Phys. Chem. 2, 233 (1991).
K. Aggarwal and J. M. Khurana, J. Mol. Struct. 1079, 21 (2015).
C. Lee, W. Yang, and R. G. Parr, J. Phys. Rev. B 37, 785 (1998).
Y. Ataly, D. Avci, and A. Basoglu, J. Struct. Chem. 19, 239 (2008).
Y. Chen, J. Wu, S. Ma, S. Zhou, X. Meng, L. Jia, and Zhiquan Pan, J. Mol. Struct. 1089, 1 (2015).
K. Fukui, Science 218, 747 (1982).
S. Gunasekaran, R. A. Balaji, S. Kumeresan, G. Anand, and S. Srinivasan, Can. J. Anal. Sci. Spectrosc. 53, 149 (2008).
D. Arul Dhas, I. Hubert Joe, S. D. D. Roy, and T. H. Freeda, Spectrochim. Acta, Part A 77, 36 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Bougharraf, H., Benallal, R., Sahdane, T. et al. Study of 5-azidomethyl-8-hydroxyquinoline structure by X-ray diffraction and HF–DFT computational methods. Russ. J. Phys. Chem. 91, 358–365 (2017). https://doi.org/10.1134/S0036024417020078
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024417020078