Abstract
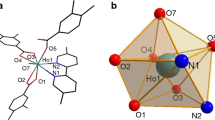
In the rare-earth element nitrate (REE)–dimethylformamide (DMF)–water systems, which can be used to obtain nanosized REE oxides by solution combustion synthesis (SCS), the formation of coordination compounds [M(H2O)3(DMF)(NO3)3]·H2O (M = La–Pr) and [M(DMF)3(NO3)3] (M = Sm–Lu, Y) has been found. Using physicochemical methods of analysis (IR spectroscopy, X-ray powder diffraction, single-crystal X-ray diffraction, elemental analysis, thermogravimetric analysis, and differential scanning calorimetry), their composition has been determined and structural features have been established; thermolysis processes have been studied in a wide temperature range. It is shown that the final products of the decomposition of complex compounds are oxides of rare earth elements.







Similar content being viewed by others
REFERENCES
I. P. Borovinskaya, A. A. Gromov, E. A. Levashov, et al., Concise Encyclopedia of Self-Propagating High-Temperature Synthesis (Elsevier, Amsterdam, 2017).
A. Varma, A. S. Mukasyan, A. S. Rogachev, and K. V. Manukyan, Chem. Rev. 116, 14493 (2016). https://doi.org/10.1021/acs.chemrev.6b00279
A. S. Mukasyan, P. Epstein, and P. Dinka, Proc. Combust. Inst. 31, 1789 (2007). https://doi.org/10.1016/j.proci.2006.07.052
S. K. Ghosh, S. N. Patra, S. K. Roy, et al., Ratio 1, 130 (2008).
A. Kumar, E. E. Wolf, and A. S. Mukasyan, Alche J. 57, 3473 (2011). https://doi.org/10.1002/aic.12537
A. J. Christy and M. Umadevi, Mater. Res. Bull. 48, 4248 (2013). https://doi.org/10.1016/j.materresbull.2013.06.072
A. Cross, S. Roslyakov, K. V. Manukyan, et al., J. Phys. Chem. 118, 26191 (2014). https://doi.org/10.1021/jp508546n
Sh. M. Khaliullin, V. D. Zhuravlev, O. V. Russkikh, et al., Int. J. Self-Propag. High-Temp. Synth. 24, 83. https://doi.org/10.3103/S106138621502003X
Z. Zhu, Y. Zhang, Y. Zhang, et al., Materials 12, 896 (2019). https://doi.org/10.3390/ma12060896
R. K. Sahu, A. K. Ray, S. K. Das, et al., J. Mater. Res. 21, 1664 (2006). https://doi.org/10.1557/jmr.2006.0211
E. V. Savinkina, I. A. Karavaev, M. S. Grigoriev, et al., Inorg. Chim. Acta 532, 120759 (2022). https://doi.org/10.1016/j.ica.2021.120759
B. M. Abu-Zied, Appl. Surf. Sci. 471, 246 (2019). https://doi.org/10.1016/j.apsusc.2018.12.007
J. J. Kingsley, N. Manickam, and K. C. Patil, Bull. Mater. Sci. 13, 179 (1990). https://doi.org/10.1007/BF02744944
A. A. Pathan, K. R. Desai, S. Vajapara, and C. P. Bhasin, Adv. Nanopart. 7, 4236 (2018).
A. A. Pathan, K. R. Desai, and C. Bhasin, Int. J. Nano. Chem. 3, 21 (2017). https://doi.org/10.18576/ijnc/030201
K. Deshpande, A. Mukasyan, and A. Varma, Chem. Mater. 16, 4896 (2004). https://doi.org/10.1021/cm040061m
J. Bai, F. Meng, C. Wei, et al., Ceram. Silik. 55, 20 (2011).
A. S. Mukasyan and P. Dinka, Int. J. Self-Propag. High-Temp. Synth. 16, 23 (2007). https://doi.org/10.3103/S1061386207010049
A. A. Voskanyan and K. Y. Chan, J. Exp. Nanosci. 6, 1080 (2015).
S. S. Krishnamurthy and S. Soundararajan, J. Inorg. Nucl. Chem. 28, 1689 (1966). https://doi.org/10.1016/0022-1902(66)80071-4
C. N. Dao, R. Rudert, P. Luger, et al., Acta Crystallogr. C48, 449 (1992). https://doi.org/10.1107/S0108270191009939
S. S. Krishnamurthy and S. Soundararajan, Can. J. Chem. 47, 995 (1969). https://doi.org/10.1139/v69-157
C. Hoch, Z. Kristallogr. Cryst. Mater. 235, 401 (2020). https://doi.org/10.1515/zkri-2020-0071
G. M. Sheldrick, SADABS. Madison, Wisconsin (USA), Bruker AXS (2008).
G. M. Sheldrick, Acta Crystallogr., Sect. A 64, 112 (2008). https://doi.org/10.1107/S0108767307043930
G. M. Sheldrick, Acta Crystallogr., Sect. C 714, 3 (2015). https://doi.org/10.1107/S2053229614024218
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds (John Wiley & Sons, Inc., Hoboken, 1997).
B. P. Hay and R. D. Hancock, Coord. Chem. Rev. 21, 61 (2001). https://doi.org/10.1016/S0010-8545(00)00366-0
B. P. Hay, O. Clement, G. Sandrone, and D. A. Dixon, Inorg. Chem. 37, 5887 (1998). https://doi.org/10.1021/ic980641j
P. E. Hansen, Molecules 26, 2409 (2021). https://doi.org/10.3390/molecules26092409
X. Shi and W. Bao, Front. Chem. 9, 723718 (2021). https://doi.org/10.3389/fchem.2021.723718
N. S. Rukk, R. S. Shamsiev, D. V. Al’bov, and S. N. Mudretsova, Fine Chem. Technol. 16, 113 (2021). https://doi.org/10.32362/2410-6593-2021-16-2-113-124
E. V. Savinkina, I. A. Karavaev, and M. S. Grigoriev, Polyhedron 192, 114875 (2020). https://doi.org/10.1016/j.poly.2020.114875
I. A. Karavaev, E. V. Savinkina, M. S. Grigor’ev, et al., Russ. J. Inorg. Chem. 67, 1178 (2022). https://doi.org/10.1134/S0036023622080186
ACKNOWLEDGMENTS
Elemental analysis was performed at the Center for Collective Use of MIREA - Russian Technological University with the support of the Ministry of Education and Science of the Russian Federation within the framework of agreement No. 075-15-2021-689 dated 01.09.2021. X-ray powder diffraction was carried out at the Center for the Collective Use of Physical Methods of Investigation at the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences. Thermal analysis was performed using the equipment of “Research Chemical and Analytical Center NRC “Kurchatov Institute” Shared Research Facilities under project’s financial support by the Russian Federation, represented by the Ministry of Science and Higher Education of the Russian Federation, Agreement No. 075-15-2023-370 dd. 22.02.2023”; single-crystal X-ray diffraction was performed at the Center for the Collective Use of Physical Research Methods of the Frimkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Supplementary Information
Figs. S1–S14. IR spectra of complexes.
Figs. S15–S28. Thermal curves of complexes.
Table S1. Assignment of frequencies in IR spectra of complexes of rare-earth nitrates with DMF.
Table S2. Selected bond lengths and bond angles for complex [Pr(H2O)3(DMF)(NO3)3]⋅H2O (III).
Table S3. Characteristics of selected hydrogen bonds in complex [Pr(H2O)3(DMF)(NO3)3]⋅H2O (III).
Table S4. Selected bond lengths and bond angles for complex [Dy(DMF)3(NO3)3] (IX).
Table S5. Selected bond lengths and bond angles for complex [Er(DMF)3(NO3)3] (XI).
Table S6. Selected bond lengths and bond angles for complex [Y(DMF)3(NO3)3] (XV).
Rights and permissions
About this article
Cite this article
Petrichko, M.I., Karavaev, I.A., Savinkina, E.V. et al. Rare-Earth Nitrate Complexes with Dimethylformamide. Russ. J. Inorg. Chem. 68, 415–423 (2023). https://doi.org/10.1134/S0036023623600193
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023623600193