Abstract
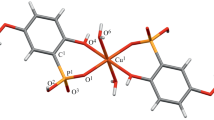
Complex [Cu(H2L)2(Н2О)2] has been obtained by the reaction of copper(II) with 2-hydroxy-5-ethylphenylphosphonic acid (H3L), and its structure was studied by X-ray diffraction. Protonation constants of acid H3L and stability constants of its complexes with Cu2+ in water have been determined by potentiometric titration. Complex [Cu(H2L)2(Н2О)2] has been found to show low toxicity and high analgesic activity.



Similar content being viewed by others
REFERENCES
L. D. Quin, A Guide To Organophosphorus Chemistry (Wiley-Interscience, New York, 2000).
Best Synthetic Methods: Organophosphorus(V) Chemistry, Ed. by C. M. Timperley (Academic Press, London, 2013).
E. De Clercq, Biochem. Pharmacol. 82, 99 (2011). https://doi.org/10.1016/j.bcp.2011.03.02
U. Pradere, E. C. Garnier-Amblard, S. J. Coats, et al., Chem. Rev. 114, 9154 (2014). https://doi.org/10.1021/cr5002035
C. Queffélec, M. Petit, P. Janvier, et al., Chem. Rev. 112, 3777 (2012). https://doi.org/10.1021/cr2004212
M. A. Shameem and A. Orthaber, Chem. - Eur. J. 22, 10718 (2016). https://doi.org/10.1002/chem.201600005
M. Dutartre, J. Bayardon, and S. Jugé, Chem. Soc. Rev. 45, 5771 (2016). https://doi.org/10.1039/C6CS00031B
I. S. Ivanova, A. B. Ilyukhin, G. S. Tsebrikova, et al., Inorg. Chim. Acta 497, 119095 (2019). https://doi.org/10.1016/j.ica.2019.119095
I. S. Ivanova, V. E. Baulin, I. N. Polyakova, et al., Russ. J. Gen. Chem. 87, 2574 (2017). https://doi.org/10.1134/S107036321711010X
M. Zhang, X. Jia, H. Zhu, et al., Org. Biomol. Chem. 17, 2972 (2019). https://doi.org/10.1039/C9OB00129H
V. E. Baulin, I. P. Kalashnikova, Y. B. Vikharev, et al., Russ. J. Gen. Chem. 88, 1786 (2018). https://doi.org/10.1134/S1070363218090049
I. S. Ivanova, V. E. Baulin, E. N. Pyatova, et al., Russ. J. Gen. Chem. 88, 1867 (2018). https://doi.org/10.1134/S1070363218090177
J. E. Weder, C. T. Dillon, T. W. Hambley, et al., Coord. Chem. Rev. 232, 95 (2002). https://doi.org/10.1016/S0010-8545(02)00086-3
E. Novak, D. W. Osborne, L. E. Matheson, et al., Drug Dev. Ind. Pharm. 17, 373 (1991). https://doi.org/10.3109/03639049109043833
T. W. Hambley, Dalton Trans. 43, 4929 (2007). https://doi.org/10.1039/b706075k
S. H. van Rijt and P. J. Sadler, Drug Discov. Today 14, 1089 (2009). https://doi.org/10.1016/j.drudis.2009.09.003
L. Ronconi and P. J. Sadler, Coord. Chem. Rev. 251, 1633 (2007). https://doi.org/10.1016/j.ccr.2006.11.017
K. H. Thompson and C. Orvig, Science 300, 936 (2003). https://doi.org/10.1126/science.1083004
M. Wehbe, A. W. Y. Leung, M. J. Abrams, et al., Dalton Trans. 46, 10758 (2017). https://doi.org/10.1039/c7dt01955f
R. Malekshah, M. Salehi, M. Kubicki, et al., J. Coord. Chem. 71, 952 (2018). https://doi.org/10.1080/00958972.2018.1447668
M. H. Sadhu, S. B. Kumar, J. K. Saini, et al., Inorg. Chim. Acta 466, 219 (2017). https://doi.org/10.1016/j.ica.2017.06.006
U. Ndagi, N. Mhlongo, and M. E. Soliman, Drug Des., Dev. Ther. 11, 599 (2017). https://doi.org/10.2147/DDDT.S119488
M. Shabbir, Z. Akhter, H. Ismail, et al., J. Mol. Struct. 1146, 57 (2017). https://doi.org/10.1016/j.molstruc.2017.05.127
Z. Piri, Z. Moradi-Shoeili, and A. Assoud, Inorg. Chem. Commun. 84, 122 (2017). https://doi.org/10.1016/j.inoche.2017.08.005
A. Jayamani, N. Sengottuvelan, and G. Chakkaravarthi, Polyhedron 81, 764 (2014). https://doi.org/10.1016/j.poly.2014.05.076
N. Uzun, A. T. Colak, F. M. Emen, et al., J. Coord. Chem. 68, 949 (2015). https://doi.org/10.1080/00958972.2014.1003371
X. Ling, C. S. Cutler, and C. J. Anderson, The Radiopharmaceutical Chemistry of the Radioisotopes of Copper (Springer Nature, Switzerland AG, 2019). https://doi.org/10.1007/978-3-319-98947-1_19
N. Bandara, A. K. Sharma, S. Krieger, et al., J. Am. Chem. Soc. 139, 12550 (2017). https://doi.org/10.1021/jacs.7b05937
M. A. Orlova, T. P. Trofimova, N. S. Zolotova, et al., Russ. Chem. Bull. Int. Ed 68, 1933 (2019). https://doi.org/10.1007/s11172-019-2649-2
L. E. McInnes, A. Noor, K. Kysenius, et al., Inorg. Chem. 58, 3382 (2019). https://doi.org/10.1021/acs.inorgchem.8b03466
S. Shuvaev, O. Kotova, V. Utochnikova, et al., Inorg. Chem. Commun. 20, 73 (2012). https://doi.org/10.1016/j.inoche.2012.02.020
S. Shuvaev, I. S. Bushmarinov, I. Sinev, et al., Eur. J. Inorg. Chem. 27, 4823 (2013). https://doi.org/10.1002/ejic.201300540
G. S. Tsebrikova, R. T. Barsamian, V. P. Solov’ev, et al., Russ. Chem. Bull. Int. Ed. 67, 2184 (2018). https://doi.org/10.1007/s11172-018-2352-8
V. P. Solov’ev, ChemEqui Software for Calculating Chemical Equilibrium Constants and Related Parameters Based on Experimental Results of Physicochemical Methods such as UV, IR and NMR Spectroscopy, Calorimetry, Potentiometry and Conductometry. http://vpsolovev.ru/programs/ (Application Date August 12, 2020).
V. P. Solov’ev and A. Y. Tsivadze, Prot. Met. Phys. Chem. Surfaces 51, 1 (2015). https://doi.org/10.1134/S2070205115010153
P. H. Müller, P. Neumann, and R. Storm, Tafeln der Mathematischen Statistik (VEB Fachbuchverlag, Leipzig, 1979).
S. Bandyopadhyay, A. Das, G. N. Mukherjee, et al., Inorg. Chim. Acta 357, 3563 (2004). https://doi.org/10.1016/j.ica.2004.05.010
M. Ali, M. Pant, and A. Abraham, Trans. Inst. Meas. Control 34, 691 (2012). https://doi.org/10.1177/0142331211403032
Bruker AXS Inc., APEX3 and SAINT 2016.
G. M. Sheldrick, SADABS, Program for Empirical Absorption Correction of Area Detector Data (1997).
G. M. Sheldrick, Acta Crystallogr., Sect. C 71, 3 (2015). https://doi.org/10.1107/S2053229614024218
M. L. Belen’kii, Elements of Quantitative Assessment of Pharmacological Effect (Meditsinskaya Literatura, Leningrad, 1963) [in Russian].
G. S. Tsebrikova, I. N. Polyakova, V. P. Solov’ev, et al., Inorg. Chim. Acta 478, 250 (2018). https://doi.org/10.1016/j.ica.2018.04.007
B. E. Baulin, M. A. Kiskin, I. S. Ivanova, et al., Russ. J. Inorg. Chem. 57, 671 (2012). https://doi.org/10.1134/S0036023612050038
V. E. Baulin, L. K. Minacheva, I. S. Ivanova, et al., Russ. J. Inorg. Chem. 56, 1222 (2011). https://doi.org/10.1134/S0036023611080043
V. E. Baulin, L. K. Minacheva, I. S. Ivanova, et al., Russ. J. Inorg. Chem. 56, 1232 (2011). https://doi.org/10.1134/S0036023611080055
S. V. Demin, S. E. Nefedov, V. E. Baulin, et al., Russ. J. Coord. Chem. 39, 333 (2013). https://doi.org/10.1134/S1070328413040052
I. N. Polyakova, V. E. Baulin, I. S. Ivanova, et al., Crystallogr. Rep. 60, 57 (2015). https://doi.org/10.1134/S1063774515010162
L. J. Bellamy, The Infra-Red Spectra of Complex Molecules (Methuen & Co. LTD, John Wiley & Sons, Inc., London–New York, 1954).
K. Nakanishi, Infrared Absorption Spectroscopy (Holden-Day, Inc., San Francisco; Nankodo Company Limited, Tokyo, 1962).
C. O. Nuallain, J. Inorg. Nucl. Chem. 36, 339 (1974). https://doi.org/10.1016/0022-1902(74)80020-5
L. H. J. Lajunen, R. Portanova, J. Piispanen, et al., Pure Appl. Chem. 69, 329 (1997). https://doi.org/10.1351/pac199769020329
G. Venkatnarayana, S. Swamy, and P. Lingaiah, Indian J. Chem. 23 (1984).
M. Puchoňová, S. Matejová, V. Jorík, et al., Polyhedron 151, 152 (2018). https://doi.org/10.1016/j.poly.2018.05.036
Y. F. Al Ansari and V. E. Baulin, Russ. J. Inorg. Chem. 64, 550 (2019). https://doi.org/10.1134/S0036023619040028
T. I. Ignat’eva, V. E. Baulin, E. N. Tsvetkov, and O. A. Raevskii, Zh. Obshch. Kh. 60, 1503 (1990).
V. Solov’ev, A. Varnek, and A. Tsivadze, J. Comput. Aided Mol. Des. 28, 549 (2014). https://doi.org/10.1007/s10822-014-9741-3
C. Doutremepuich, Thrombosis 2012, 626289 (2012). https://doi.org/10.1155/2012/626289
C. M. Turnbull, A. G. Rossi, and I. L. Megson, Expert Opin. Ther. Targets 10, 911 (2006). https://doi.org/10.1517/14728222.10.6.911
F. Buttgereit, G. R. Burmester, and L. S. Simon, Am. J. Med. 110, 13 (2001). https://doi.org/10.1016/s0002-9343(00)00728-2
T. Jacka, C. C. A. Bernard, and G. Singer, Life Sci. 32, 1023 (1983). https://doi.org/10.1016/0024-3205(83)90934-7
M. O’Connor, A. Kellett, M. McCann, et al., J. Med. Chem. 55, 1957 (2012). https://doi.org/10.1021/jm201041d
Funding
This work was performed under the State Assignment for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Institute of Physiologically Active Substances, Russian Academy of Sciences (project no. 0090-2019-0008) and with partial financial support of the Russian Science Foundation (project nos. 19-13-00294 (constants computations) and 21-43-00020 (testing of biological activity)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Additional information
Translated by I. Kudryavtsev
Rights and permissions
About this article
Cite this article
Ivanova, I.S., Tsebrikova, G.S., Rogacheva, Y.I. et al. Complexing Properties of 2-Hydroxy-5-Ethylphenylphosphonic Acid (H3L). Crystal Structure and Analgesic Activity of [Cu(H2L)2(Н2О)2]. Russ. J. Inorg. Chem. 66, 1846–1853 (2021). https://doi.org/10.1134/S0036023621120068
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621120068