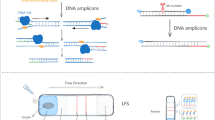
Abstract
A diagnostic system based on recombinase polymerase amplification (RPA) has been developed to identify six bacterial pathogens of human pneumonia. Species-specific primers have been designed and optimized to conduct a multiplex reaction in one common volume. Labeled primers were used for reliable discrimination of amplification products that are similar in size. Identification of the pathogen was carried out by visual analysis of an electrophoregram. The analytical sensitivity of the developed multiplex RPA was 102–103 copies of DNA. The specificity of the system was determined by the absence of cross-amplification of the studied DNA samples of pneumonia pathogens for each pair of primers, as well as for the DNA of Mycobacterium tuberculosis H37rv, and amounted to 100%. The execution time of the analysis is less than an 1 h, including the electrophoretic reaction control. The test system can be used in specialized clinical laboratories for rapid analysis of samples from patients with suspected pneumonia.

Similar content being viewed by others
REFERENCES
Campigotto A., Mubareka S. 2015. Influenza-associated bacterial pneumonia; managing and controlling infection on two fronts. Expert. Rev. Anti. Infect. Ther. 13, 55–68.
Noskin G.A., Glassroth J. 1996. Bacterial pneumonia associated with HIV-1 infection. Clin. Chest. Med. 17, 713–723.
Henig O., Kaye K.S. 2017. Bacterial pneumonia in older adults. Infect. Dis. Clin. North Am. 31, 689–713.
Eshwara V.K., Mukhopadhyay C., Rello J. 2020. Community-acquired bacterial pneumonia in adults: an update. Indian J. Med. Res. 151, 287–302.
Harris M., Clark J., Coote N., Fletcher P., Harnden A., McKean M., Thomson A. 2011. British thoracic society standards of care committee. British thoracic society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 66 (suppl. 2), ii1–ii23.
Cunha B.A. 2006. The atypical pneumonias: clinical diagnosis and importance. Clin. Microbiol. Infect. 12 (suppl. 3), 12–24.
Azoulay E., Russell L., Van de Louw A., Metaxa V., Bauer P., Povoa P., Montero J.G., Loeches I.M., Mehta S., Puxty K., Schellongowski P., Rello J., Mokart D., Lemiale V., Mirouse A. 2020. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med. 46, 298–314.
Mabie M., Wunderink R.G. 2003. Use and limitations of clinical and radiologic diagnosis of pneumonia. Semin. Respir. Infect. 18, 72–79.
Budayanti N.S., Suryawan K., Iswari I.S., Sukrama D.M. 2019. The quality of sputum specimens as a predictor of isolated bacteria from patients with lower respiratory tract infections at a tertiary referral hospital, Denpasar, Bali-Indonesia. Front. Med. (Lausanne). 6, 64.
Lee N., Rainer T.H., Ip M., Zee B., Ng M.H., Antonio G.E., Chan E., Lui G., Cockram C.S., Sung J.J., Hui D.S. 2006. Role of laboratory variables in differentiating SARS-coronavirus from other causes of community-acquired pneumonia within the first 72 h of hospitalization. Eur. J. Clin. Microbiol. Infect. Dis. 25, 765–772.
Dorigo-Zetsma J.W., Zaat S.A., Wertheim-van Dillen P.M., Spanjaard L., Rijntjes J., van Waveren G., Jensen J.S., Angulo A.F., Dankert J. 1999. Comparison of PCR, culture, and serological tests for diagnosis of Mycoplasma pneumoniae respiratory tract infection in children. J. Clin. Microbiol. 37, 14–17.
Lapa S.A., Klochikhina E.S., Miftakhov R.A., Zolotov A.M., Zasedatelev A.S., Chudinov A.V. 2020. Multiplex PCR for identification of bacterial pathogens of infectious pneumonia. Russ. J. Bioorg. Chem. 46 (5), 859–861.
Lapa S.A., Klochikhina E.S., Miftakhov R.A., Zasedatelev A.S., Chudinov A.V. 2021. Development of multi-primer PCR system with an open architecture for rapid detection of infectious pneumonia causative agents. AIP Conf. Proc. 2388, 030018.
Piepenburg O., Williams C.H., Stemple D.L., Armes N.A. 2006. DNA detection using recombination proteins. PLoS Biol. 4, e204.
Lobato I.M., O’Sullivan C.K. 2018. Recombinase polymerase amplification: basics, applications and recent advances. Trends Anal. Chem. 98, 19–35.
Wilson K. 2001. Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. 56, 2.4.1–2.4.5.
Lapa S.A., Miftakhov R.A., Klochikhina E.S., Sh-ershov V.E., Zasedatelev A.S., Chudinov A.V., Ammur Y.I., Blagodatskikh S.A. 2021. Development of multiplex RT-PCR with immobilized primers for identification of infectious human pneumonia pathogens. Mol. Biol. (Moscow). 55 (6), 828‒838. https://doi.org/10.1134/S0026893321040063
Klochikhina E.S., Shershov V.E., Kuznetsova V.E., Lapa S.A., Chudinov A.V. 2021. Specificities of multi-primer polymerase chain reaction optimization for the detection of infectious pneumonia agents in human. Fine Chem. Technol. 16 (3), 225‒231.
Funding
The study was supported by a grant Russian Science Foundation (no. 22-14-00257).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain a description of any research involving humans or animals as subjects.
Rights and permissions
About this article
Cite this article
Lapa, S.A., Surzhikov, S.A., Blagodatskikh, S.A. et al. Recombinase Polymerase Amplification for Rapid Detection of Human Bacterial Pneumonia Pathogens. Mol Biol 57, 544–549 (2023). https://doi.org/10.1134/S0026893323030068
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893323030068