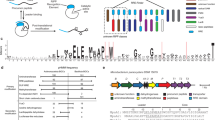
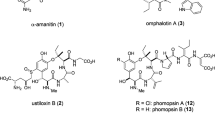
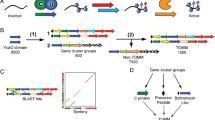
Abstract
A great variety of prokaryotic ribosomally synthesized and posttranslationally modified peptides (RiPPs) were predicted and identified experimentally owing to recent advances in large-scale genome sequencing and data analysis. Thiazole/oxazole-modified microcins (TOMMs) are a group of RiPPs that have characteristic thiazole and oxazole heterocycles derived from cysteine and serine residues. The review summarizes the available data on the classification, structure, and biosynthesis of TOMMs and discusses their biological activity and potential use in medicine.
Similar content being viewed by others
Abbreviations
- LAP:
-
linear azol(in)e-containing peptide
- RiPP:
-
ribosomally synthesized and posttranslationally modified peptide
- TOMM:
-
thiazole/oxazole-modified microcin
- SLS:
-
streptolysin S
References
Arnison P.G., Bibb M.J., Bierbaum G., Bowers A.A., Bugni T.S., Bulaj G., Camarero J.A., Campopiano D.J., Challis G.L., Clardy J., Cotter P.D., Craik D.J., Dawson M., Dittmann E., Donadio S., Dorrestein P.C., Entian K.D., Fischbach M.A., Garavelli J.S., Goransson U., Gruber C.W., Haft D.H., Hemscheidt T.K., Hertweck C., Hill C., Horswill A.R., Jaspars M., Kelly W.L., Klinman J.P., Kuipers O.P., Link A.J., Liu W., Marahiel M.A., Mitchell D.A., Moll G.N., Moore B.S., Muller R., Nair S.K., Nes I.F., Norris G.E., Olivera B.M., Onaka H., Patchett M.L., Piel J., Reaney M.J., Rebuffat S., Ross R.P., Sahl H.G., Schmidt E.W., Selsted M.E., Severinov K., Shen B., Sivonen K., Smith L., Stein T., Sussmuth R.D., Tagg J.R., Tang G.L., Truman A.W., Vederas J.C., Walsh C.T., Walton J.D., Wenzel S.C., Willey J.M., van der Donk W.A. 2013. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nature Prod. Rep. 30, 108–160.
Lee S.W., Mitchell D.A., Markley A.L., Hensler M.E., Gonzalez D., Wohlrab A., Dorrestein P.C., Nizet V., Dixon J.E. 2008. Discovery of a widely distributed toxin biosynthetic gene cluster. Proc. Natl. Acad. Sci. U. S. A. 105, 5879–5884.
Oman T.J., van der Donk W.A. 2010. Follow the leader: The use of leader peptides to guide natural product biosynthesis. Nature Chem. Biol. 6, 9–18.
Zhang W., Li Y., Qian G., Wang Y., Chen H., Li Y.Z., Liu F., Shen Y., Du L. 2011. Identification and characterization of the anti-methicillin-resistant Staphylococcus aureus WAP-8294A2 biosynthetic gene cluster from Lysobacter enzymogenes OH11. Antimicrob. Agents Chemother. 55, 5581–5589.
Gruber C.W., Cemazar M., Anderson M.A., Craik D.J. 2007. Insecticidal plant cyclotides and related cystine knot toxins. Toxicon. 49, 561–675.
Kaas Q., Westermann J.C., Craik D.J. 2010. Conopeptide characterization and classifications: An analysis using ConoServer. Toxicon. 55, 1491–1509.
Chatterjee C., Miller L.M., Leung Y.L., Xie L., Yi M., Kelleher N.L., van der Donk W.A. 2005. Lacticin 481 synthetase phosphorylates its substrate during antibiotic production. J. Am. Chem. Soc. 127, 15332–15333.
Kelly W.L., Pan L., Li C. 2009. Thiostrepton biosynthesis: Prototype for a new family of bacteriocins. J. Am. Chem. Soc. 131, 4327–4334.
Onaka H., Nakaho M., Hayashi K., Igarashi Y., Furumai T. 2005. Cloning and characterization of the goadsporin biosynthetic gene cluster from Streptomyces sp. TP-A0584. Microbiology. 151, 3923–3933.
Melby J.O., Nard N.J., Mitchell D.A. 2011. Thiazole/oxazole-modified microcins: Complex natural products from ribosomal templates. Curr. Opin. Chem. Biol. 15, 369–378.
Lee J., McIntosh J., Hathaway B.J., Schmidt E.W. 2009. Using marine natural products to discover a protease that catalyzes peptide macrocyclization of diverse substrates. J. Am. Chem. Soc. 131, 2122–2124.
Li Y.M., Milne J.C., Madison L.L., Kolter R., Walsh C.T. 1996. From peptide precursors to oxazole and thiazolecontaining peptide antibiotics: Microcin B17 synthase. Science. 274, 1188–1193.
Milne J.C., Roy R.S., Eliot A.C., Kelleher N.L., Wokhlu A., Nickels B., Walsh C.T. 1999. Cofactor requirements and reconstitution of microcin B17 synthetase: A multienzyme complex that catalyzes the formation of oxazoles and thiazoles in the antibiotic microcin B17. Biochemistry. 38, 4768–4781.
Dunbar K.L., Melby J.O., Mitchell D.A. 2012. YcaO domains use ATP to activate amide backbones during peptide cyclodehydrations. Nature Chem. Biol. 8, 569–575.
Haft D.H., Basu M.K., Mitchell D.A. 2010. Expansion of ribosomally produced natural products: A nitrile hydratase- and Nif11-related precursor family. BMC Biol. 8, 70.
Velasquez J.E., van der Donk W.A. 2011. Genome mining for ribosomally synthesized natural products. Curr. Opin. Chem. Biol. 15, 11–21.
Yorgey P., Lee J., Kordel J., Vivas E., Warner P., Jebaratnam D., Kolter R. 1994. Posttranslational modifications in microcin B17 define an additional class of DNA gyrase inhibitor. Proc. Natl. Acad. Sci. U. S. A. 91, 4519–4523.
Garrido M.C., Herrero M., Kolter R., Moreno F. 1988. The export of the DNA replication inhibitor Microcin B17 provides immunity for the host cell. EMBO J. 7, 1853–1862.
Sinha Roy R., Kelleher N.L., Milne J.C., Walsh C.T. 1999. In vivo processing and antibiotic activity of microcin B17 analogs with varying ring content and altered bisheterocyclic sites. Chem. Biol. 6, 305–318.
Zamble D.B., McClure C.P., Penner-Hahn J.E., Walsh C.T. 2000. The McbB component of microcin B17 synthetase is a zinc metalloprotein. Biochemistry. 39, 16190–16199.
Ghilarov D., Serebryakova M., Shkundina I., Severinov K. 2011. A major portion of DNA gyrase inhibitor microcin B17 undergoes an N,O-peptidyl shift during synthesis. J. Biol. Chem. 286, 26308–26318.
Allali N., Afif H., Couturier M., van Melderen L. 2002. The highly conserved TldD and TldE proteins of Escherichia coli are involved in microcin B17 processing and in CcdA degradation. J. Bacteriol. 184, 3224–3231.
Heddle J.G., Blance S.J., Zamble D.B., Hollfelder F., Miller D.A., Wentzell L.M., Walsh C.T., Maxwell A. 2001. The antibiotic microcin B17 is a DNA gyrase poison: Characterisation of the mode of inhibition. J. Mol. Biol. 307, 1223–1234.
Pierrat O.A., Maxwell A. 2003. The action of the bacterial toxin microcin B17. Insight into the cleavagereligation reaction of DNA gyrase. J. Biol. Chem. 278, 35016–35023.
Pierrat O.A., Maxwell A. 2005. Evidence for the role of DNA strand passage in the mechanism of action of microcin B17 on DNA gyrase. Biochemistry. 44, 4204–4215.
Metelev M., Serebryakova M., Ghilarov D., Zhao Y., Severinov K. 2013. Microcin-B-like compounds produced by Pseudomonas syringae: Structure and species-specificity of antibacterial action. J. Bacteriol. 195(18), 4129–4137. doi 10.1128/JB.00665-13
Severinov K., Semenova E., Kazakov A., Kazakov T., Gelfand M.S. 2007. Low-molecular-weight posttranslationally modified microcins. Mol. Microbiol. 65, 1380–1394.
Cunningham M.W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13, 470–511.
Todd E.W. 1938. The differentiation of two distinct serological varieties of streptolysin, streptolysin O and streptolysin S. J. Pathol. Bacteriol. 47, 423–445. doi 10.1002/path.1700470307
Molloy E.M., Cotter P.D., Hill C., Mitchell D.A., Ross R.P. 2011. Streptolysin S-like virulence factors: The continuing sagA. Nature Rev. Microbiol. 9, 670–681.
Betschel S.D., Borgia S.M., Barg N.L., Low D.E., De Azavedo J.C. 1998. Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect. Immun. 66, 1671–1679.
Datta V., Myskowski S.M., Kwinn L.A., Chiem D.N., Varki N., Kansal R.G., Kotb M., Nizet V. 2005. Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection. Mol. Microbiol. 56, 681–695.
Gonzalez D.J., Lee S.W., Hensler M.E., Markley A.L., Dahesh S., Mitchell D.A., Bandeira N., Nizet V., Dixon J.E., Dorrestein P.C. 2010. Clostridiolysin S, a post-translationally modified biotoxin from Clostridium botulinum. J. Biol. Chem. 285, 28220–28228.
Chen X.H., Koumoutsi A., Scholz R., Eisenreich A., Schneider K., Heinemeyer I., Morgenstern B., Voss B., Hess W.R., Reva O., Junge H., Voigt B., Jungblut P.R., Vater J., Sussmuth R., Liesegang H., Strittmatter A., Gottschalk G., Borriss R. 2007. Comparative analysis of the complete genome sequence of the plant growthpromoting bacterium Bacillus amyloliquefaciens FZB42. Nature Biotechnol. 25, 1007–1014.
Scholz R., Molohon K.J., Nachtigall J., Vater J., Markley A.L., Sussmuth R.D., Mitchell D.A., Borriss R. 2011. Plantazolicin, a novel microcin B17/streptolysin S-like natural product from Bacillus amyloliquefaciens FZB42. J. Bacteriol. 193, 215–224.
Kalyon B., Helaly S.E., Scholz R., Nachtigall J., Vater J., Borriss R., Sussmuth R.D. 2011. Plantazolicin A and B: Structure elucidation of ribosomally synthesized thiazole/oxazole peptides from Bacillus amyloliquefaciens FZB42. Org. Lett. 13, 2996–2999.
Molohon K.J., Melby J.O., Lee J., Evans B.S., Dunbar K.L., Bumpus S.B., Kelleher N.L., Mitchell D.A. 2011. Structure determination and interception of biosynthetic intermediates for the plantazolicin class of highly discriminating antibiotics. ACS Chem. Biol. 6, 1307–1313.
Onaka H., Tabata H., Igarashi Y., Sato Y., Furumai T. 2001. Goadsporin, a chemical substance which promotes secondary metabolism and morphogenesis in streptomycetes: 1. Purification and characterization. J. Antibiot. (Tokyo). 54, 1036–1044.
Onaka H. 2009. Biosynthesis of indolocarbazole and goadsporin, two different heterocyclic antibiotics produced by actinomycetes. Biosci. Biotechnol. Biochem. 73, 2149–2155.
Donia M.S., Ravel J., Schmidt E.W. 2008. A global assembly line for cyanobactins. Nature Chem. Biol. 4, 341–343.
Schmidt E.W., Donia M.S. 2009. Chapter 23. Cyanobactin ribosomally synthesized peptides: A case of deep metagenome mining. Methods Enzymol. 458, 575–596.
Schmidt E.W., Nelson J.T., Rasko D.A., Sudek S., Eisen J.A., Haygood M.G., Ravel J. 2005. Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella. Proc. Natl. Acad. Sci. U. S. A. 102, 7315–7320.
Agarwal V., Pierce E., McIntosh J., Schmidt E.W., Nair S.K. 2012. Structures of cyanobactin maturation enzymes define a family of transamidating proteases. Chem. Biol. 19, 1411–1422.
Jian X.H., Pan H.X., Ning T.T., Shi Y.Y., Chen Y.S., Li Y., Zeng X.W., Xu J., Tang G.L. 2012. Analysis of YM-216391 biosynthetic gene cluster and improvement of the cyclopeptide production in a heterologous host. ACS Chem. Biol. 7, 646–651.
Sivonen K., Leikoski N., Fewer D.P., Jokela J. 2010. Cyanobactins: Ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 86, 1213–1225.
Ishida K., Matsuda H., Murakami M., Yamaguchi K. 1997. Kawaguchipeptin B, an antibacterial cyclic undecapeptide from the cyanobacterium Microcystis aeruginosa. J. Nature Prod. 60, 724–726.
Williams A.B., Jacobs R.S. 1993. A marine natural product, patellamide D, reverses multidrug resistance in a human leukemic cell line. Cancer Lett. 71, 97–102.
Salvatella X., Caba J.M., Albericio F., Giralt E. 2003. Solution structure of the antitumor candidate trunkamide A by 2D NMR and restrained simulated annealing methods. J. Org. Chem. 68, 211–215.
Leikoski N., Fewer D.P., Jokela J., Alakoski P., Wahlsten M., Sivonen K. 2012. Analysis of an inactive cyanobactin biosynthetic gene cluster leads to discovery of new natural products from strains of the genus Microcystis. PLoS ONE. 7, e43002.
Donia M.S., Schmidt E.W. 2011. Linking chemistry and genetics in the growing cyanobactin natural products family. Chem. Biol. 18, 508–519.
McIntosh J.A., Donia M.S., Nair S.K., Schmidt E.W. 2011. Enzymatic basis of ribosomal peptide prenylation in cyanobacteria. J. Am. Chem. Soc. 133, 13698–13705.
Leikoski N., Fewer D.P., Jokela J., Wahlsten M., Rouhiainen L., Sivonen K. 2010. Highly diverse cyanobactins in strains of the genus Anabaena. Appl. Environ. Microbiol. 76, 701–709.
Donia M.S., Hathaway B.J., Sudek S., Haygood M.G., Rosovitz M.J., Ravel J., Schmidt E.W. 2006. Natural combinatorial peptide libraries in cyanobacterial symbionts of marine ascidians. Nature Chem. Biol. 2, 729–735.
McIntosh J.A., Robertson C.R., Agarwal V., Nair S.K., Bulaj G.W., Schmidt E.W. 2010. Circular logic: Nonribosomal peptide-like macrocyclization with a ribosomal peptide catalyst. J. Am. Chem. Soc. 132, 15499–15501.
McIntosh J.A., Schmidt E.W. 2010. Marine molecular machines: Heterocyclization in cyanobactin biosynthesis. ChemBioChem. 11, 1413–1421.
McIntosh J.A., Donia M.S., Schmidt E.W. 2010. Insights into heterocyclization from two highly similar enzymes. J. Am. Chem. Soc. 132, 4089–4091.
Sudek S., Haygood M.G., Youssef D.T., Schmidt E.W. 2006. Structure of trichamide, a cyclic peptide from the bloom-forming cyanobacterium Trichodesmium erythraeum, predicted from the genome sequence. Appl. Environ. Microbiol. 72, 4382–4387.
Fu X., Do T., Schmitz F.J., Andrusevich V., Engel M.H. 1998. New cyclic peptides from the ascidian Lissoclinum patella. J. Nat. Prod. 61, 1547–1551.
Bagley M.C., Dale J.W., Merritt E.A., Xiong X. 2005. Thiopeptide antibiotics. Chem. Rev. 105, 685–714.
Carnio M.C., Holtzel A., Rudolf M., Henle T., Jung G., Scherer S. 2000. The macrocyclic peptide antibiotic micrococcin P(1) is secreted by the food-borne bacterium Staphylococcus equorum WS 2733 and inhibits Listeria monocytogenes on soft cheese. Appl. Environ. Microbiol. 66, 2378–2384.
Fuller A.T. 1955. A new antibiotic of bacterial origin. Nature. 175, 722.
Su T.L. 1948. Micrococcin, an antibacterial substance formed by a strain of Micrococcus. Br. J. Exp. Pathol. 29, 473–781.
Dutcher J.D., Vandeputte J. 1955. Thiostrepton, a new antibiotic: 2. Isolation and chemical characterization. Antibiot. Annu. 3, 560–561.
Liao R., Duan L., Lei C., Pan H., Ding Y., Zhang Q., Chen D., Shen B., Yu Y., Liu W. 2009. Thiopeptide biosynthesis featuring ribosomally synthesized precursor peptides and conserved posttranslational modifications. Chem. Biol. 16, 141–147.
Heffron S.E., Jurnak F. 2000. Structure of an EF-Tu complex with a thiazolyl peptide antibiotic determined at 2.35 — bition of EF-Tu. Biochemistry. 39, 37–45.
Parmeggiani A., Krab I.M., Okamura S., Nielsen R.C., Nyborg J., Nissen P. 2006. Structural basis of the action of pulvomycin and GE2270A on elongation factor Tu. Biochemistry. 45, 6846–6857.
Hensens O.D., Albers-Schönberg G. 1978. Total structure of the peptide antibiotic components of thiopeptin by 1H and 13C NMR spectroscopy. Tetrahedron Lett. 19, 3649–3652.
Puar M.S., Ganguly A.K., Afonso A., Brambilla R., Mangiaracina P., Sarre O., MacFarlane R.D. 1981. Sch 18640, a new thiostrepton-type antibiotic. J. Am. Chem. Soc. 103, 5231–5233.
Nishimura H., Kimura T., Tawara K., Sasaki K., Nakajima K., Shimaoka N., Okamoto S., Shimohira M., Isono J. 1957. Aburamycin, a new antibiotic. J. Antibiot. (Tokyo). 10, 205–212.
Puar M.S., Chan T.M., Hegde V., Patel M., Bartner P., Ng K.J., Pramanik B.N., MacFarlane R.D. 1998. Sch 40832, a novel thiostrepton from Micromonospora carbonacea. J. Antibiot. (Tokyo). 51, 221–224.
Benazet F., Cartier M., Florent J., Godard C., Jung G., Lunel J., Mancy D., Pascal C., Renaut J., Tarridec P., Theilleux J., Tissier R., Dubost M., Ninet L. 1980. Nosiheptide, a sulfur-containing peptide antibiotic isolated from Streptomyces actuosus 40037. Experientia. 36, 414–416.
Lentzen G., Klinck R., Matassova N., Aboul-ela F., Murchie A.I. 2003. Structural basis for contrasting activities of ribosome binding thiazole antibiotics. Chem. Biol. 10, 769–778.
Porse B.T., Cundliffe E., Garrett R.A. 1999. The antibiotic micrococcin acts on protein L11 at the ribosomal GTPase centre. J. Mol. Biol. 287, 33–45.
Baumann S., Schoof S., Bolten M., Haering C., Takagi M., Shin-ya K., Arndt H.D. 2010. Molecular determinants of microbial resistance to thiopeptide antibiotics. J. Am. Chem. Soc. 132, 6973–6981.
Rodnina M.V., Savelsbergh A., Matassova N.B., Katunin V.I., Semenkov Y.P., Wintermeyer W. 1999. Thiostrepton inhibits the turnover but not the GTPase of elongation factor G on the ribosome. Proc. Natl. Acad. Sci. U. S. A. 96, 9586–9590.
Harms J.M., Wilson D.N., Schluenzen F., Connell S.R., Stachelhaus T., Zaborowska Z., Spahn C.M., Fucini P. 2008. Translational regulation via L11: Molecular switches on the ribosome turned on and off by thiostrepton and micrococcin. Mol. Cell. 30, 26–38.
Anborgh P.H., Parmeggiani A. 1991. New antibiotic that acts specifically on the GTP-bound form of elongation factor Tu. EMBO J. 10, 779–784.
Mizuhara N., Kuroda M., Ogita A., Tanaka T., Usuki Y., Fujita K. 2011. Antifungal thiopeptide cyclothiazomycin B1 exhibits growth inhibition accompanying morphological changes via binding to fungal cell wall chitin. Bioorg. Med. Chem. 19, 5300–5310.
Aoki M., Ohtsuka T., Yamada M., Ohba Y., Yoshizaki H., Yasuno H., Sano T., Watanabe J., Yokose K., Seto H. 1991. Cyclothiazomycin, a novel polythiazole-containing peptide with renin inhibitory activity. Taxonomy, fermentation, isolation and physico-chemical characterization. J. Antibiot. (Tokyo). 44, 582–588.
Morris R.P., Leeds J.A., Naegeli H.U., Oberer L., Memmert K., Weber E., LaMarche M.J., Parker C.N., Burrer N., Esterow S., Hein A.E., Schmitt E.K., Krastel P. 2009. Ribosomally synthesized thiopeptide antibiotics targeting elongation factor Tu. J. Am. Chem. Soc. 131, 5946–5955.
Yu Y., Duan L., Zhang Q., Liao R., Ding Y., Pan H., Wendt-Pienkowski E., Tang G., Shen B., Liu W. 2009. Nosiheptide biosynthesis featuring a unique indole side ring formation on the characteristic thiopeptide framework. ACS Chem. Biol. 4, 855–864.
Wieland Brown L.C., Acker M.G., Clardy J., Walsh C.T., Fischbach M.A. 2009. Thirteen posttranslational modifications convert a 14-residue peptide into the antibiotic thiocillin. Proc. Natl. Acad. Sci. U. S. A. 106, 2549–2553.
Wang J., Yu Y., Tang K., Liu W., He X., Huang X., Deng Z. 2010. Identification and analysis of the biosynthetic gene cluster encoding the thiopeptide antibiotic cyclothiazomycin in Streptomyces hygroscopicus 10–22. Appl. Environ. Microbiol. 76, 2335–2344.
Bowers A.A., Walsh C.T., Acker M.G. 2010. Genetic interception and structural characterization of thiopeptide cyclization precursors from Bacillus cereus. J. Am. Chem. Soc. 132, 12182–12184.
Young T.S., Walsh C.T. 2011. Identification of the thiazolyl peptide GE37468 gene cluster from Streptomyces ATCC 55365 and heterologous expression in Streptomyces lividans. Proc. Natl. Acad. Sci. U. S. A. 108, 13053–13058.
Takahashi Y., Naganawa H., Takita T., Umezawa H., Nakamura S. 1976. The revised structure of bottromycin A2. J. Antibiot. (Tokyo). 29, 1120–1123.
Schipper D. 1983. The revised structure of bottromycin A2. J. Antibiot. (Tokyo). 36, 1076–1077.
Shimamura H., Gouda H., Nagai K., Hirose T., Ichioka M., Furuya Y., Kobayashi Y., Hirono S., Sunazuka T., Omura S. 2009. Structure determination and total synthesis of bottromycin A2: A potent antibiotic against MRSA and VRE. Angew. Chem. Int. Ed. Engl. 48, 914–917.
Gouda H., Kobayashi Y., Yamada T., Ideguchi T., Sugawara A., Hirose T., Omura S., Sunazuka T., Hirono S. 2012. Three-dimensional solution structure of bottromycin A2: A potent antibiotic active against methicillin-resistant Staphylococcus aureus and vancomycinresistant enterococci. Chem. Pharm. Bull. (Tokyo). 60, 169–171.
Waisvisz J.M., van der Hoeven M.G., Nijenhuis B.t. 1957. The structure of the sulfur-containing moiety of bottromycin. J. Am. Chem. Soc. 79, 4524–4527.
Tokuyama H., Yokoshima S., Yamashita T., Fukuyama T. 1998. A novel ketone synthesis by a palladium-catalyzed reaction of thiol esters and organozinc reagents. Tetrahedron Lett. 39, 3189–3192.
Kobayashi Y., Ichioka M., Hirose T., Nagai K., Matsumoto A., Matsui H., Hanaki H., Masuma R., Takahashi Y., Omura S., Sunazuka T. 2010. Bottromycin derivatives: Efficient chemical modifications of the ester moiety and evaluation of anti-MRSA and anti-VRE activities. Bioorg. Med. Chem. Lett. 20, 6116–6120.
Otaka T., Kaji A. 1976. Mode of action of bottromycin A2. Release of aminoacyl- or peptidyl-tRNA from ribosomes. J. Biol. Chem. 251, 2299–2306.
Otaka T., Kaji A. 1981. Mode of action of bottromycin A2: Effect on peptide bond formation. FEBS Lett. 123, 173–176.
Otaka T., Kaji A. 1983. Mode of action of bottromycin A2: Effect of bottromycin A2 on polysomes. FEBS Lett. 153, 53–59.
Huo L., Rachid S., Stadler M., Wenzel S.C., Muller R. 2012. Synthetic biotechnology to study and engineer ribosomal bottromycin biosynthesis. Chem. Biol. 19, 1278–1287.
Crone W.J.K., Leeper F.J., Truman A.W. 2012. Identification and characterisation of the gene cluster for the anti-MRSA antibiotic bottromycin: expanding the biosynthetic diversity of ribosomal peptides. Chemical Sci. 3, 3516–3521.
Gomez-Escribano J.P., Song L., Bibb M.J., Challis G.L. 2012. Posttranslational β-methylation and macrolactamidination in the biosynthesis of the bottromycin complex of ribosomal peptide antibiotics. Chemical Sci. 3, 3522–3525.
Hou Y., Tianero M.D., Kwan J.C., Wyche T.P., Michel C.R., Ellis G.A., Vazquez-Rivera E., Braun D.R., Rose W.E., Schmidt E.W., Bugni T.S. 2012. Structure and biosynthesis of the antibiotic bottromycin D. Org Lett. 14, 5050–5053.
Tanaka N., Nishimura T., Nakamura S., Umezawa H. 1966. Biological studies on bottromycin A and its hydrazide. J. Antibiot. (Tokyo). 19, 149–154.
Inoue M., Shinohara N., Tanabe S., Takahashi T., Okura K., Itoh H., Mizoguchi Y., Iida M., Lee N., Matsuoka S. 2010. Total synthesis of the large nonribosomal peptide polytheonamide B. Nature Chem. 2, 280–285.
McIntosh J.A., Donia M.S., Schmidt E.W. 2009. Ribosomal peptide natural products: Bridging the ribosomal and nonribosomal worlds. Nature Prod. Rep. 26, 537–559.
Wang H., Fewer D.P., Sivonen K. 2011. Genome mining demonstrates the widespread occurrence of gene clusters encoding bacteriocins in cyanobacteria. PLoS ONE. 6, e22384.
Kersten R.D., Yang Y.L., Xu Y., Cimermancic P., Nam S.J., Fenical W., Fischbach M.A., Moore B.S., Dorrestein P.C. 2011. A mass spectrometry-guided genome mining approach for natural product peptidogenomics. Nature Chem. Biol. 7, 794–802.
Freeman M.F., Gurgui C., Helf M.J., Morinaka B.I., Uria A.R., Oldham N.J., Sahl H.G., Matsunaga S., Piel J. 2012. Metagenome mining reveals polytheonamides as posttranslationally modified ribosomal peptides. Science. 338, 387–390.
Deane C.D., Melby J.O., Molohon K.J., Susarrey A.R., Mitchell D.A. 2013. Engineering unnatural variants of plantazolicin through codon reprogramming. ACS Chem. Biol. 8(9), 1998–2008. doi 10.1021/cb4003392
Lee J., Hao Y., Blair P.M., Melby J.O., Agarwal V., Burkhart B.J., Nair S.K., Mitchell D.A. 2013. Structural and functional insight into an unexpectedly selective N-methyltransferase involved in plantazolicin biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 110, 12954–12959.
Dunbar K.L., Mitchell D.A. 2013. Insights into the mechanism of peptide cyclodehydrations achieved through the chemoenzymatic generation of amide derivatives. J. Am. Chem. Soc. 135, 8692–8701.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.V. Metelev, D.A. Ghilarov, 2014, published in Molekulyarnaya Biologiya, 2014, Vol. 48, No. 1, pp. 36–54.
Rights and permissions
About this article
Cite this article
Metelev, M.V., Ghilarov, D.A. Structure, function, and biosynthesis of thiazole/oxazole-modified microcins. Mol Biol 48, 29–45 (2014). https://doi.org/10.1134/S0026893314010105
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893314010105