Abstract
A study of a series of single-atom-alloy catalysts Pd1Ag3/Al2O3, Pd1Ag3/CeO2–Al2O3, and Pd1Ag3/CeO2–ZrO2 in the selective hydrogenation of diphenylacetylene (DPA) showed a significant (five-fold) increase in activity for the PdAg3/CeO2–ZrO2 sample in comparison with that of Pd1Ag3/Al2O3. It was especially noted that the increase in activity was not accompanied by a decrease in the selectivity for the target product. This catalytic behavior can be explained by two factors: (1) a more than twofold increase in the dispersity of the PdAg3/CeO2–ZrO2 catalyst and (2) a change in the electronic state of the nanoparticles, as determined from the results of an IR-spectroscopic study of adsorbed CO. The retention of the high selectivity of the synthesized catalysts indicated the stability of the structure of Pd1 monoatomic sites in the catalysts prepared by deposition on Ce-containing supports, which was also confirmed by the IR spectroscopy of adsorbed CO. The experimental results indicate that Ce-containing supports are promising for the synthesis of catalysts for the selective hydrogenation of substituted alkynes.
Similar content being viewed by others
INTRODUCTION
The selective hydrogenation of alkynes is one of the key and most important reactions in modern catalysis [1–4]. The large-scale selective hydrogenation of acetylenic hydrocarbons in pyrolysis ethylene or styrene before their polymerization are illustrative examples of the industrial use of this reaction [1, 5, 6]. The application of the selective hydrogenation of alkynes to fine organic synthesis makes it possible to obtain cis- and trans-olefins as feedstock for the food and pharmaceutical (for example, the synthesis of vitamins A, E, and K) industries and for the production of solvents, detergents, etc. [7].
Normally, palladium is used as an active component in selective hydrogenation catalysts; it ensures high activity, but the selectivity of the process is often insufficient [8, 9]. In the past few years the concept of single-atom alloy (SAA) catalysts has been actively studied as a promising direction in the development of highly selective catalysts. [10–12]. In these systems, active catalytic sites are Pd1 atoms isolated from each other by modifier metal atoms (M = Ag, Au, Zn, or In), whose activity in hydrogenation is negligible. The use of single-atom-alloy catalysts provides an extremely high selectivity for the target product; however, as a rule, their activity is insignificant due to a small number of isolated sites on the surface of Pd–M nanoparticles. Therefore, to increase the activity is a problem of considerable current interest.
The catalyst activity can be significantly affected by a support, which also contributes to the activity/selectivity ratio [13, 14]. An important parameter when choosing a support is the degree of interaction between metal nanoparticles and the support surface. It is well known that all supports can be divided into inert (weakly interacting with supported metals) and active (strongly interacting with a supported active phase). In the latter case, both a change in the electronic state of surface metal particles and an increase in their dispersity are possible [15, 16].
In recent years, great interest of researchers has been attracted by cerium oxide. Its using as a support makes it possible to increase the dispersion of a supported metal and to vary the electron density on metal nanoparticles [5, 13, 17]. In some cases, sites on the periphery of nanoparticles at the metal–support interface, which include both the nanoparticle metal atoms and the surface atoms of the support, can play an important role. To date, a significant increase in catalytic activity has been found for a number of supported metal catalysts obtained using cerium oxide, although the reasons for this increase still remain unclear. An increased activity of Pd/CeO2 was found in the hydrogenation of phenol [18, 19]. It was hypothesized that an increase in the dispersity of Pd in the catalyst supported on CeO2 can facilitate an increase in the catalytic activity in the conversion of phenol into cyclohexanone.
Note that CeO2 is often used in combination with other oxides. The structural and textural characteristics of cerium oxide can be significantly improved by using zirconium dioxide as a modifier. According to published data [20, 21], the addition of even small amounts of ZrO2 makes it possible to increase the thermal stability CeO2 as a result of the formation of a CeO2–ZrO2 solid solution.
Redina and coauthors [22–25] observed an increase in the activity of a monometallic Pt catalyst in the hydrogenation of various organic oxygen- and nitrogen-containing substrates when Pt was deposited on a CeO2–ZrO2 support. They suggested that the high catalytic activity of the mesoporous Pt/CeO2–ZrO2 catalyst was caused by active hydrogen spillover, which results in partial reduction of the CeO2–ZrO2 carrier with the formation of sites for the of adsorption and activation of an initial substrate. In addition, in the hydrogenation of cinnamic aldehyde, the dependence of the yield of unsaturated alcohol on the surface area of supports for the synthesized Pt catalysts was noted.
Similar results in the hydrogenation of cinnamaldehyde were obtained previously for Pt/CeO2–ZrO2 [26, 27] and Pd/CeO2–ZrO2 [28]. In all cases, the samples based on CeO2–ZrO2 outperformed reference catalysts in terms of activity and selectivity.
In the above examples, an increase in the activity effect was found for catalysts containing monometallic nanoparticles. Unfortunately, the possibility of increasing the activity of bimetallic catalysts supported on CeO2–ZrO2 remains almost unexplored. In this regard, we can only mention the work by Bhogeswararao et al. [29], who studied the hydrogenation of cinnamaldehyde on modified Ni–M/CeO2–ZrO2 (M = Pt, Pd) catalysts.
It should be noted that there are no published data on the activity of single-atom alloy catalysts on Ce-containing supports. In connection with the foregoing, the aim of this work was to study the effect of CeO2–ZrO2 and CeO2–Al2O3 supports on the activity of single-atom alloy Pd1Ag3 catalysts in the hydrogenation of diphenylacetylene (DPA). In this case, special attention was paid to studying the effect of the support on the catalyst selectivity. Previously, a similar PdAg3/Al2O3 catalyst showed extremely high selectivity in the hydrogenation of DPA [30, 31] and 1-phenyl-1-propyne [31, 32], but its activity was significantly lower than that of a monometallic counterpart.
To determine the influence of the support on the activity/selectivity ratio of single-atom alloy catalysts, we prepared a series of Pd1Ag3/CeO2–ZrO2, Pd1Ag3/CeO2–Al2O3, and Pd1Ag3/Al2O3 samples and studied their structural characteristics using the IR spectroscopy of adsorbed CO and high-resolution transmission electron microscopy. The elemental composition of the synthesized catalysts was evaluated from the data of energy dispersive spectroscopy. The catalytic characteristics were studied in the liquid-phase hydrogenation of DPA.
EXPERIMENTAL
Catalyst Preparation
All samples were obtained by the co-impregnation impregnation of supports with Pd(NO3)2 and AgNO3 solutions. The supports used were γ-Al2O3 (Sasol, Germany; SBET = 56 m2/g), 20% CeO2–80% Al2O3 (Puralox SCFa-160 Ce20, Sasol, Germany), and 80% CeO2–20% ZrO2 (C20Z, OOO Ekoal’yans, Sverdlovsk oblast, Novouralsk, Russia). Before loading the active component, all of the supports were preliminarily calcined in an air flow at 550°C for 4 h. After the impregnation, the samples were dried at room temperature and then calcined in a flow of air (300 mL/min) at 550°C and reduced in a flow of 5% H2/Ar at 550°C. Upon completion of the reduction, the samples were cooled to 200°C in a flow of 5% H2/Ar and then to room temperature in a flow of high-purity N2. The metal content of the finished catalysts was 2 wt % Pd and 6 wt % Ag. In this paper, the catalysts are designated as follows: Pd1Ag3/Al2O3 as PdAg3/A, Pd1Ag3/CeO2–Al2O3 as PdAg3/CA, and Pd1Ag3/CeO2–ZrO2 as PdAg3/CZ.
Catalyst Characterization
Transmission electron microscopy (TEM). The structure and microstructure of the samples were studied by high-resolution transmission electron microscopy (HRTEM) on a ThemisZ electron microscope (Thermo Fisher Scientific, USA) with an accelerating voltage of 200 kV and a limiting resolution of 0.07 nm. Images were recorded using a Ceta 16 CCD array (Thermo Fisher Scientific, USA). The instrument was equipped with a SuperX energy-dispersive characteristic X-ray spectrometer (EDX) (Thermo Fisher Scientific, USA) with a semiconductor Si detector with an energy resolution of 128 eV.
For electron-microscopic studies, sample particles were deposited from an alcohol solution onto perforated carbon substrates fixed on copper grids using an UZD-1UCh2 ultrasonic disperser (Russia), which made it possible to achieve a uniform distribution of particles over the substrate surface.
IR spectroscopy of adsorbed CO. The diffuse reflectance IR spectra of adsorbed CO were recorded using a Tensor 27 IR spectrometer (Bruker, Germany) with a Harrick Diffuse Reflectance Kit for in situ measurements (Harrick Scientific Products, the United Kingdom). A weighed sample portion (~0.02 g) was placed in a cell with CaF2 glasses and heated in a flow of Ar to 550°С; thereafter, the sample was reduced in a flow of 5% H2/Ar for 1 h. Next, the sample was first cooled to 300°С in a flow of 5% H2/Ar and then to 50°С in a flow of Ar, and the background spectrum was recorded. The spectra of adsorbed CO were recorded at 50°С in a flow of 0.5 vol % CO/He for 20 min (250 scans; resolution, 4 cm–1).
Liquid-Phase Hydrogenation of Diphenylacetylene
The hydrogenation was carried out in an autoclave-type catalytic setup. A weighed portion of the catalyst was loaded into a glass reactor with a substrate and a solvent. The reactor was placed in an autoclave equipped with a magnetic stirrer, an electronic pressure sensor, and a gas supply and sampling system. The process was carried out at a temperature of 25°C and an initial hydrogen pressure of 5 bar with constant stirring. Diphenylacetylene (98%; Sigma-Aldrich, Germany) was used as a substrate, and n-hexane (>99%; Merck, Germany) was used as a solvent. In order to correctly compare the results, the reaction was carried out in the kinetic mode. The reaction products were analyzed by gas chromatography on a Kristall 5000 chromatograph (Khromatek, Russia) equipped with a flame-ionization detector using an HP5-MS chromatographic column (5% phenyldimethylsiloxane) 30 m long, with an internal diameter of 0.25 mm, and a stationary phase film thickness of 0.25 µm. Based on the results of the gas-chromatographic analysis of a reaction mixture, the selectivity toward target diphenylethylene formation (S=) was calculated as follows:
where n= and n– are the molar fractions of the resulting alkene and alkane, respectively.
The efficiency of the synthesized catalysts was evaluated by the catalyst turnover frequencies (TOFs) at the first (TOF1) and second (TOF2) stages of hydrogenation based on the reaction rates at these stages. The specific activity values were calculated based on the total number of palladium atoms in the catalyst sample because it is difficult to determine the number of surface Pd atoms in bimetallic PdAg catalysts using electron microscopy.
Considering that the hydrogenation reaction can proceed in two stages (hydrogenation of the starting alkyne to an olefin and its subsequent hydrogenation to an alkane), the reaction rates were calculated for the first and second stages (r1 and r2, respectively). The values of r1 and r2 were determined from the slopes of curves in the plots of the amount of absorbed hydrogen versus reaction time: at the first stage, in a range of 0.2–0.6 equiv of absorbed H2 for all catalysts and, at the second stage, in ranges of 1.0–1.4 and 1.0–1.2 equiv for PdAg3/CZ and PdAg3/CA or PdAg3/A, respectively. The rate of hydrogen uptake was found in terms of 1 g of the catalyst (mmol H2 min–1 gCat–1). The ratio between the rates of alkyne and alkene hydrogenation (r1/r2) was used to evaluate the efficiency of kinetic control of the process.
RESULTS AND DISCUSSION
Mapping Transmission Electron Microscopy
Figure 1 presents the results of the HRTEM study of PdAg3/CZ, PdAg3/CA, and PdAg3/A catalysts, mapping data for the main components of the catalysts, and histograms of the size distributions of bimetallic PdAg particles. A wide particle size distribution (from 1 to 12 nm) was observed for all of the samples, while the average sizes were 3.5, 5.4, and 9.2 nm for PdAg3/CZ, PdAg3/CA, and PdAg3/A, respectively. It can be clearly seen that all particles had a spherical shape. The EDX mapping of different parts of the catalyst samples made it possible to conclude that the PdAg particles were uniformly distributed over the support surface and the Ag/Pd ratio corresponded to the calculated one. The method proposed by Ichikawa et al. [33] was used to calculate the dispersity of bimetallic PdAg particles, which was 0.32, 0.21, or 0.12 for PdAg3/CZ, PdAg3/CA, and PdAg3/A, respectively.
TEM data illustrating the location of PdAg particles on the support surface: (a) for PdAg3/CZ with mapping for (b) Pd, Ag, and Ce; (c) Pd; and (d) Ag; (e) for PdAg3/CA with mapping for (f) Pd, Ag, Ce, and Al; (g) Pd; and (h) Ag; and (i) for PdAg3/A with mapping for (k) Pd, Ag, Al; (l) Pd; and (m) Ag. Figs. 1a, 1e, and 1i show the corresponding particle-size distribution histograms.
IR Spectroscopy of Adsorbed CO
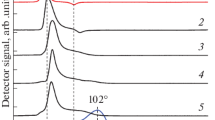
The surface structure of the synthesized catalysts was studied by the IR spectroscopy of adsorbed CO (Fig. 2). Two absorption bands were detected in the spectrum of PdAg3/CZ: a high-intensity band with a maximum at 2055 cm–1 characteristic of CO molecules adsorbed on the catalyst surface in a linear form and a marginal band (at the noise level) at 1964 cm–1, which can be attributed to CO adsorption in a bridged form (Fig. 2, spectrum 1). Similar spectra were also observed for the PdAg3/CA and PdAg3/A catalysts. In the case of PdAg3/CA, the absorption bands related to the adsorption of CO in linear and bridged forms were detected at 2045 and 1953 cm–1 (Fig. 2, spectrum 2). For PdAg3/A, these signals were identified at 2047 and 1960 cm–1, respectively (Fig. 2, spectrum 3). The extremely low intensity of the absorption bands in a range of 1953–1964 cm–1 almost completely excluded the formation of sites for the multipoint adsorption of CO molecules on the surface of PdAg nanoparticles [34, 35] and indicated the formation of a PdAg alloy with Pd1 atoms as active sites isolated from each other by Ag atoms. As a result of the formation of bimetallic PdAg particles, the electron density on palladium atoms can increase to cause an increase in the electron density donation to the π antibonding orbital of the adsorbed CO molecule and a weakening of the C–O bond [34, 35].
It should be noted that the symmetry and small width of the absorption band of linearly adsorbed CO also indicate the formation of highly uniform Pd1 active sites in the test catalysts.
Selective Hydrogenation of Diphenylacetylene
The main goal of this work was to study the possibility of increasing the activity of a single-atom alloy PdAg3 catalyst with the retention of high selectivity. Figure 3 shows the kinetic curves of hydrogen uptake in the hydrogenation of DPA over these catalysts. Table 1 systematizes data on the rates of hydrogenation and the turnover frequencies at the first and second stages of the reaction. The PdAg3/A catalyst exhibited the lowest catalytic activity in DPA hydrogenation; for this catalyst, the rate r1 was 5.41 mmol H2 min–1 \({\text{g}}_{{{\text{Cat}}}}^{{ - 1}}\). The hydrogenation rate of the initial DPA on the PdAg3 catalyst obtained by depositing the active component on CeO2–Al2O3 (PdAg3/CA) was slightly higher (7.32 mmol H2 min–1 \({\text{g}}_{{{\text{Cat}}}}^{{ - 1}}\)). When CeO2–ZrO2 was used as a support (the PdAg3/CZ catalyst), the rate sharply increased and reached 28.76 mmol H2 min–1 \({\text{g}}_{{{\text{Cat}}}}^{{ - 1}}\). The experimental data are in good agreement with those published previously for the hydrogenation of organic oxygen- and nitrogen-containing substrates over Pt/CeO2–ZrO2 and Pd/CeO2–ZrO2 catalysts [23, 25–28], and allowed us to conclude that the activity of single-atom alloy PdAg catalysts can be increased by supporting bimetallic nanoparticles onto CeO2–ZrO2.
The activity of catalysts may be affected by the size of bimetallic nanoparticles [36]. Thus, the lowest catalytic activity in the case of PdAg3/A probably stems from the formation of larger (to 9.2 nm) bimetallic particles, as compared to other catalysts of this series (cf. Figs. 1a, 1e, and 1i). On going from PdAg3/A to the samples obtained by deposition onto a cerium-containing support, the average particle size decreased to 5.4 nm (PdAg3/CA) and 3.5 nm (PdAg3/CZ). It is likely that this pronounced decrease in the size of bimetallic PdAg particles was associated with an increase in the energy of metal–support interaction for PdAg3/CZ. Thus, it was shown in a number of publications that the presence of Zr ions in the crystal lattice of cerium oxide enhances the metal–support interaction between the support and nanoparticles deposited on it, which can lead to a higher dispersity of metal particles [27, 37, 38]. This conclusion is in good agreement with the experimental TEM data.
Note that the calculation using the formula proposed by Ichikawa et al. [33] showed that the dispersity value for PdAg3/CZ was higher by a factor of only 2.6 than that for PdAg3/A (see the Mapping Transmission Electron Microscopy section). Thus the higher catalytic activity of the PdAg3/CZ catalyst can be only partly explained by an increase in the dispersity with consideration for the fact that the reaction rates for these catalysts differed from each other by a factor of 5.
In part, the activity of PdAg3/CZ can increase due to a change in the electronic state of nanoparticles as a result of the metal–support interaction [39]. In the IR spectra of adsorbed CO (Fig. 2), the band of CO adsorbed in a linear form on the PdAg3/CZ sample shifted toward higher wavenumbers from 2045 to 2055 cm–1 in comparison with those for PdAg3/CA and PdAg3/A. This can be caused by the formation of Ce3+ acidic sites that occur when zirconium is introduced into the cerium structure. As a result of interaction with Ce3+ sites, the electron density decreases on PdAg nanoparticles, which become more electron-deficient. In turn, an increase in the degree of electron deficiency of PdAg nanoparticles can contribute to an increase in the activity of the catalyst in the hydrogenation. This assumption is consistent with a review published earlier by Stakheev and Kustov [39], who considered the influence of a support on the electronic properties and catalytic characteristics of supported metal catalysts.
After the uptake of 1 equiv of hydrogen, the kinetic curves of all samples showed a characteristic bend (Fig. 3), which indicates a slowdown in the hydrogenation of the triple C≡C bond to an olefin intermediate [40, 41]. The calculation of the hydrogenation rates performed using kinetic data showed a significant decrease in the value of r2 in comparison with r1 (Table 1). The rates of hydrogenation on the PdAg3/A and PdAg3/CA samples decreased from 5.41 to 0.14 and from 7.32 to 0.30 mmol H2 min–1 \({\text{g}}_{{{\text{Cat}}}}^{{ - 1}}\), respectively. In the case of the PdAg3/CZ catalyst, the rate decreased from 28.76 to 2.07 mmol H2 min–1 \({\text{g}}_{{{\text{Cat}}}}^{{ - 1}}\). This significant slowdown of the reaction after the completion of C≡C bond hydrogenation contributed to effective kinetic control of the reaction.
It is interesting to compare changes in the composition of products with reaction time for the PdAg3/CZ and PdAg3/A catalysts (Fig. 4). In the hydrogenation products, cis- and trans-diphenylethylenes and diphenylethane were detected. The given dependences illustrate the conclusion on a significant difference in the rates of DPA hydrogenation on different catalysts: for example, a DPA conversion of 100% on the PdAg3/CZ or PdAg3/A sample was reached in 15 or 80 min, respectively. For both catalysts, diphenylethane was present in trace amounts at the very beginning of the reaction, and the alkane content in the reaction products was no higher than 7–8% upon reaching a DPA conversion of 100%. The maximum yield of the target olefin for both PdAg3/CZ and PdAg3/A was 89%. The experimental data indicate that the selectivity of the catalysts was almost identical.
For a more detailed analysis of the selectivity, we studied its dependency on the conversion of initial DPA (Fig. 5). We found that, regardless of the support, the selectivity of all of the studied samples was ~96–98% in almost the entire range of conversions. At the same time, upon reaching a DPA conversion of 100%, the selectivity of the catalysts on a Ce-containing support (~96%) was slightly higher than that of the catalyst supported on Al2O3 (~93%).
The high selectivity of bimetallic PdAg systems can be explained by the migration of Ag atoms to the surface of the PdAg alloy after high-temperature activation of catalysts with hydrogen, resulting in the formation of Pd1 sites isolated by Ag atoms [42]. This also led to a predominant decrease in the adsorption energy of an olefin, which facilitated its desorption and prevented the subsequent undesirable hydrogenation to an alkane.
It is likely that the high selectivity of bimetallic catalysts with Ce-containing supports is retained due to the retention of the structure of isolated Pd1 sites upon the preparation of nanoparticles. This is evidenced by the data of the IR spectroscopy of adsorbed CO, which indicate the domination of the linearly adsorbed CO characteristic of the CO adsorption on Pd1 sites isolated from each other by Ag atoms (on which CO is not adsorbed at room temperature) (Fig. 2). The signal of bridging CO adsorption band was insignificant or almost completely absent, which excludes the possibility of the formation of multipoint CO adsorption sites on the surface of PdAg nanoparticles. This conclusion is in good agreement with the results published by Tang et al. [43]. The effect of an increase in selectivity as a result of the formation of monoatomic Pd1 sites on the surface of bimetallic nanoparticles was found by a number of authors for the selective hydrogenation of acetylene to ethylene [44, 45] and in our previous studies [30, 31, 42].
CONCLUSIONS
The results obtained in this work indicate that the use of Ce-containing supports can significantly increase the activity of single-atom PdAg catalysts in the selective hydrogenation of substituted alkynes to the corresponding alkenes. For example, PdAg3/CZ prepared using a CeO2–ZrO2 mixed oxide exhibited a fivefold increase in the rate of reaction, as compared to that on the catalyst prepared based on traditional Al2O3. It is extremely important that the increase in activity was not accompanied by a decrease in selectivity toward formation of the target product.
The increase in the activity of Ce-containing catalysts can partly be explained by an almost twofold increase in the dispersity, which led to an increase in the surface area of bimetallic nanoparticles. In addition, according to the data of the IR spectroscopy of adsorbed CO, the metal–support interaction, which changes the electronic state of PdAg nanoparticles, can play a role in increasing the activity. Thus high selectivity is retained due to the stability of the structure of single-atom Pd1 sites on the surface of PdAg nanoparticles deposited on Ce-containing supports, as evidenced by the results of the IR spectroscopy of adsorbed CO.
REFERENCES
Glyzdova, D.V., Smirnova, N.S., Shlyapin, D.A., and Tsyrul’nikov, P.G., Russ. J. Gen. Chem., 2020, vol. 90, p. 1120.
Sun, Z., Wang, S., and Chen, W., J. Mater. Chem. A, 2021, vol. 9, p. 5296.
Zhang, L., Zhou, M., Wang, A., and Zhang, T., Chem. Rev., 2020, vol. 120, p. 683.
Ravanchi, M., Sahebdelfar, S., and Komeili, S., Rev. Chem. Eng., 2017, vol. 34, p. 215.
Shittu, T.D. and Ayodele, O.B., Front. Chem. Sci. Eng., 2022, vol. 16. p. 1031.
Zhao, X., Chang, Y., Chen, W.-J., Wu, Q., Pan, X., Chen, K., and Weng, B., ACS Omega, 2022, vol. 7, p. 17.
Chen, X., Shi, C., and Liang, C., Chin. J. Catal., 2021, vol. 42, p. 2105.
Borodziski, A. and Bond, G.C., Catal. Rev., 2006, vol. 48, p. 91.
Borodziski, A. and Bond, G.C., Catal. Rev., 2008, vol. 50, p. 379.
Han, Z.-K., Sarker, D., Ouyang, R., Mazheika, A., Gao, Y., and Levchenko, S.V., Nat. Commun., 2021, vol. 12, no. 1833. https://doi.org/10.1038/s41467-021-22048-9
Han, J., Lu, J., Wang, M., Wang, Y., and Wang, F., Chin. J. Chem., 2019, vol. 37, p. 977.
Giannakakis, G., Flytzani-Stephanopoulos, M., and Sykes, E.C.H., Acc. Chem. Res., 2019, vol. 52, p. 237.
Razmgar, K., Altarawneh, M., Oluwoye, I., and Senanayake, G.,Catal. Surv. Asia, 2021, vol. 25, p. 27.
Pakhomov, N.A., Nauchnye osnovy prigotovleniya katalizatorov: vvedenie v teoriyu i praktiku (Scientific Fundamentals of Preparation of Catalysts: Introduction to Theory and Practice), Sadykov, V.A., Ed., Novosibirsk: SB RAS, 2011.
Abdel-Mageed, A.M., Chen, S., Fauth, C., Haring, T., and Bansmann, J., ChemPhysChem, 2021, vol. 22, p. 1302.
Schubert, M.M., Hackenberg, S., van Veen, A.C., Muhler, M., Plzakc, V., and Jürgen Behm, R., J. Catal., 2001, vol. 197, p. 113.
Anand, S., Pinheiro, D., and Sunaja Devi, K.R., Asian J. Org. Chem., 2021, vol. 10, p. 3068.
Nelson, N.C., Manzano, J.S., Sadow, A.D., Overbury, S.H., and Slowing, I.I., ACS Catal., 2015, vol. 5, p. 2051.
Velu, S., Kapoor, M.P., Inagaki, S., and Suzuki, K., Appl. Catal., A, 2003, vol. 245, p. 317.
Zhang, X., Wang, Q., Zhang, J., Wang, J., Guo, M., Chen, S., Li, C., Hu, C., and Xie, Y., RSC Adv., 2015, vol. 5, p. 89976.
Teng, M., Luo, L., and Yang, X., Microporous Mesoporous Mater., 2009, vol. 119, p. 158.
Vikanova, K.V. and Redina, E.A., Russ. J. Phys. Chem. A, 2018, vol. 92, p. 2355.
Vikanova, K.V., Redina, E.A., Kapustin, G.I., Davshan, N.A., and Kustov, L.M., Russ. J. Phys. Chem. A, 2019, vol. 93, p. 231.
Vikanova, K.V. and Redina, E.A., Russ. J. Phys. Chem. A, 2018, vol. 92, p. 2374.
Redina, E.A., Vikanova, K.V., Kapustin, G.I., Mishin, I.V., Tkachenko, O.P., and Kustov, L.M., Eur. J. Org. Chem., 2019, p. 4159.
Bhogeswararao, S. and Srinivas, D., J. Catal. 2012, vol. 285, p. 31.
Wei, S., Zhao, Y., Fan, G., Yang, L., and Li, F., Chem. Eng. J. 2017, vol. 322, p. 234.
Bhogeswararao, S. and Srinivas, D., Catal. Lett. 2010, vol. 140, p. 55.
Bhogeswararao, S., Pavan Kumar, V., Chary, K.V.R., and Srinivas, D., Catal. Lett., 2013, vol. 143, p. 1266.
Rassolov, A.V., Mashkovsky, I.S., Bragina, G.O., Baeva, G.N., Markov, P.V., Smirnova, N.S., Wärnå, J., Stakheev, A.Yu., and Murzin, D.Yu., Mol. Catal., 2021, vol. 506, no. 111550. https://doi.org/10.1016/j.mcat.2021.111550
Rassolov, A.V., Bragina, G.O., Baeva, G.N., Mashkovskii, I.S., and Stakheev, A.Yu., Kinet. Catal., 2020, vol. 61, p. 869.
Rassolov, A.V., Mashkovsky, I.S., Baeva, G.N., Bragina, G.O., Smirnova, N.S., Markov, P.V., Bukhtiyarov, A.V., Wärnå, J., Stakheev, A.Yu, and Murzin, D.Yu., Nanomaterials, 2021, vol. 11, no. 3286. https://doi.org/10.3390/nano11123286
Ichikawa, S., Poppa, H., and Boudart, M., J. Catal., 1985, vol. 91, p. 1.
Rassolov, A.V., Bragina, G.O., Baeva, G.N., Smirnova, N.S., Kazakov, A.V., Mashkovsky, I.S., Bukhtiyarov, A.V., Zubavichus, Ya.V., and Stakheev, A.Yu., Kinet. Catal., 2020, vol. 61, p. 758.
Rassolov, A.V., Bragina, G.O., Baeva, G.N., Smirnova, N.S., Kazakova, A.V., Mashkovskii, I.S., and Stakheev, A.Yu., Kinet. Catal., 2019, vol. 60, p. 642.
Nikolaev, S.A., Zanaveskin, L.N., Smirnov, V.V., Aver’yanov, V.A., and Zanaveskin, K.L., Russ. Chem. Rev., 2009, vol. 78, p. 231.
Alifanti, M., Baps, B., Blangenois, N., Naud, J., Grange, P., and Delmon, B., Chem. Mater., 2003, vol. 15, p. 395.
Liang, Q., Wu, X., Wu, X., and Weng, D., Catal. Lett., 2007, vol. 119, p. 265.
Stakheev, A.Yu. and Kustov, L.M., Appl. Catal., A, 1999, vol. 188, p. 3.
Choudary, B.M., Lakshmi Kantam, M., Mahender Reddy, N., Koteswara Rao, K., Haritha, Y., Bhaskar, V., Figueras, F., and Tuel, A., Appl. Catal., A, 1999, vol. 181, p. 139.
Marín-Astorga, N., Alvez-Manoli, G., and Reyes, P., J. Mol. Catal. A: Chem., 2005, vol. 226, p. 81.
Pei, G.X., Liu, X.Y., Wang, A., Lee, A.F., Isaacs, M.A., Li, L., Pan, X., Yang, X., Wang, X., Tai, Z., Wilson, K., and Zhang, T., ACS Catal., 2015, vol. 5, p. 3717.
Tang, J., Deng, L., Deng, H., Xiao, S., Zhang, X., and Hu, W., J. Phys. Chem. C, 2014, vol. 118, p. 27850.
Jin, Y., Datye, A.K., Rightor, E., Gulotty, R., Waterman, W., Smith, M., Holbrook, M., and Maj, J., J. Catal., 2001, vol. 203, p. 292.
Sheth, P.A., Neurock, M., and Smith, C.M., J. Phys. Chem. B, 2005, vol. 109, p. 12449.
Funding
This work was supported by the Russian Science Foundation (RSF grant no. 19-13-00285-P).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Abbreviations and notation: DPA, diphenylacetylene; HRTEM, high resolution transmission electron microscopy; EDX, energy dispersive X-ray spectroscopy; TOF, catalyst turnover frequency; r, the rate of reaction.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rassolov, A.V., Bragina, G.O., Baeva, G.N. et al. Highly Active Bimetallic Single-Atom Alloy PdAg Catalysts on Cerium-Containing Supports in the Hydrogenation of Alkynes to Alkenes. Kinet Catal 63, 756–764 (2022). https://doi.org/10.1134/S0023158422060118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158422060118