Abstract
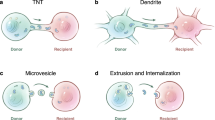
Recently described phenomenon of intercellular transfer of mitochondria attracts the attention of researchers in both fundamental science and translational medicine. In particular, the transfer of mitochondria results in the initiation of stem cell differentiation, in reprogramming of differentiated cells, and in the recovery of the lost mitochondrial function in recipient cells. However, the mechanisms of mitochondria transfer between cells and conditions inducing this phenomenon are studied insufficiently. It is still questionable whether this phenomenon exists in vivo. Moreover, it is unclear, how the transfer of mitochondria into somatic cells is affected by the ubiquitination system that, for example, is responsible for the elimination of “alien” mitochondria of the spermatozoon in the oocyte during fertilization. Studies on these processes can provide a powerful incentive for development of strategies for treatment of mitochondria-associated pathologies and give rise a new avenue for therapeutic approaches based on “mitochondrial transplantation”.
Similar content being viewed by others
Abbreviations
- iPS:
-
induced pluripotent stem cells
- MMSC:
-
multipotent mesenchymal stromal cell
- mtDNA:
-
mitochondrial DNA
- TNT:
-
tunneling nanotube
References
Weiss, P. A., and Mayr, R. (1971) Neuronal organelles in neuroplasmic (“axonal”) flow. I. Mitochondria, Acta Neuropathol., 5,Suppl. 5, 187–197.
Zorov, D. B., Isaev, N. K., Plotnikov, E. Y., Zorova, L. D., Stelmashook, E. V., Vasileva, A. K., Arkhangelskaya, A. A., and Khryapenkova, T. G. (2007) The mitochondrion as Janus bifrons, Biochemistry (Moscow), 72, 1115–1126.
Amchenkova, A. A., Bakeeva, L. E., Chentsov, Y. S., Skulachev, V. P., and Zorov, D. B. (1988) Coupling membranes as energy-transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes, J. Cell Biol., 107, 481–495.
Leterrier, J. F., Rusakov, D. A., Nelson, B. D., and Linden, M. (1994) Interactions between brain mitochondria and cytoskeleton: evidence for specialized outer membrane domains involved in the association of cytoskeleton-associated proteins to mitochondria in situ and in vitro, Microsc. Res. Tech., 27, 233–261.
Chang, D. T., and Reynolds, I. J. (2006) Mitochondrial trafficking and morphology in healthy and injured neurons, Prog. Neurobiol., 80, 241–268.
Baloyannis, S. J. (2006) Mitochondrial alterations in Alzheimer’s disease, J. Alzheimer’s Dis., 9, 119–126.
Rogers, R. S., and Bhattacharya, J. (2013) When cells become organelle donors, Physiology (Bethesda), 28, 414–422.
Gerdes, H. H., Bukoreshtliev, N. V., and Barroso, J. F. (2007) Tunneling nanotubes: a new route for the exchange of components between animal cells, FEBS Lett., 581, 2194–2201.
Rustom, A., Saffrich, R., Markovic, I., Walther, P., and Gerdes, H. H. (2004) Nanotubular highways for intercellular organelle transport, Science, 303, 1007–1010.
Bukoreshtliev, N. V., Wang, X., Hodneland, E., Gurke, S., Barroso, J. F., and Gerdes, H. H. (2009) Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells, FEBS Lett., 583, 1481–1488.
Gurke, S., Barroso, J. F., Hodneland, E., Bukoreshtliev, N. V., Schlicker, O., and Gerdes, H. H. (2008) Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells, Exp. Cell Res., 314, 3669–3683.
Onfelt, B., Nedvetzki, S., Yanagi, K., and Davis, D. M. (2004) Cutting edge: membrane nanotubes connect immune cells, J. Immunol., 173, 1511–1513.
Schiller, C., Huber, J. E., Diakopoulos, K. N., and Weiss, E. H. (2013) Tunneling nanotubes enable intercellular transfer of MHC class I molecules, Hum. Immunol., 74, 412–416.
Galkina, S. I., Molotkovsky, J. G., Ullrich, V., and Sud’ina, G. F. (2005) Scanning electron microscopy study of neutrophil membrane tubulovesicular extensions (cytonemes) and their role in anchoring, aggregation and phagocytosis. The effect of nitric oxide, Exp. Cell Res., 304, 620–629.
Freund, D., Bauer, N., Boxberger, S., Feldmann, S., Streller, U., Ehninger, G., Werner, C., Bornhauser, M., Oswald, J., and Corbeil, D. (2006) Polarization of human hematopoietic progenitors during contact with multipotent mesenchymal stromal cells: effects on proliferation and clonogenicity, Stem Cells Dev., 15, 815–829.
Koyanagi, M., Brandes, R. P., Haendeler, J., Zeiher, A. M., and Dimmeler, S. (2005) Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes, Circ. Res., 96, 1039–1041.
Plotnikov, E. Y., Khryapenkova, T. G., Vasileva, A. K., Marey, M. V., Galkina, S. I., Isaev, N. K., Sheval, E. V., Polyakov, V. Y., Sukhikh, G. T., and Zorov, D. B. (2008) Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture, J. Cell Mol. Med., 12, 1622–1631.
Plotnikov, E. Y., Khryapenkova, T. G., Galkina, S. I., Sukhikh, G. T., and Zorov, D. B. (2010) Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture, Exp. Cell Res., 316, 2447–2455.
Marzo, L., Gousset, K., and Zurzolo, C. (2012) Multifaceted roles of tunneling nanotubes in intercellular communication, Front. Physiol., 72, 1–14.
Arkwright, P. D., Luchetti, F., Tour, J., Roberts, C., Ayub, R., Morales, A. P., Rodriguez, J. J., Gilmore, A., Canonico, B., Papa, S., and Esposti, M. D. (2010) Fas stimulation of T lymphocytes promotes rapid intercellular exchange of death signals via membrane nanotubes, Cell Res., 20, 72–88.
Ahmad, T., Mukherjee, S., Pattnaik, B., Kumar, M., Singh, S., Kumar, M., Rehman, R., Tiwari, B. K., Jha, K. A., Barhanpurkar, A. P., Wani, M. R., Roy, S. S., Mabalirajan, U., Ghosh, B., and Agrawal, A. (2014) Miro1 regulates intercellular mitochondrial transport and enhances mesenchymal stem cell rescue efficacy, EMBO J., 33, 994–1010.
Lou, E., Fujisawa, S., Morozov, A., Barlas, A., Romin, Y., Dogan, Y., Gholami, S., Moreira, A. L., Manova-Todorova, K., and Moore, M. A. (2012) Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma, PLoS One, 7, e33093.
Kadiu, I., and Gendelman, H. E. (2011) Macrophage bridging conduit trafficking of HIV-1 through the endoplasmic reticulum and Golgi network, J. Proteome Res., 10, 3225–3238.
Domhan, S., Ma, L., Tai, A., Anaya, Z., Beheshti, A., Zeier, M., Hlatky, L., and Abdollahi, A. (2011) Intercellular communication by exchange of cytoplasmic material via tunneling nanotube-like structures in primary human renal epithelial cells, PLoS One, 6, e21283.
Wang, Y., Cui, J., Sun, X., and Zhang, Y. (2011) Tunnelingnanotube development in astrocytes depends on p53 activation, Cell Death Differ., 18, 732–742.
He, K., Shi, X., Zhang, X., Dang, S., Ma, X., Liu, F., Xu, M., Lv, Z., Han, D., Fang, X., and Zhang, Y. (2011) Longdistance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes, Cardiovasc. Res., 92, 39–47.
Lou, E., Fujisawa, S., Barlas, A., Romin, Y., Manova-Todorova, K., Moore, M. A., and Subramanian, S. (2012) Tunneling nanotubes: a new paradigm for studying intercellular communication and therapeutics in cancer, Commun. Integr. Biol., 5, 399–403.
Chinnery, H. R., Pearlman, E., and McMenamin, P. G. (2008) Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea, J. Immunol., 180, 5779–5783.
Islam, M. N., Das, S. R., Emin, M. T., Wei, M., Sun, L., Westphalen, K., Rowlands, D. J., Quadri, S. K., Bhattacharya, S., and Bhattacharya, J. (2012) Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury, Nature Med., 18, 759–765.
Wu, X. S., Masedunskas, A., Weigert, R., Copeland, N. G., Jenkins, N. A., and Hammer, J. A. (2012) Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes, Proc. Natl. Acad. Sci. USA, 109, 2101–2109.
Bisharyan, Y., and Clark, T. G. (2011) Calcium-dependent mitochondrial extrusion in ciliated protozoa, Mitochondrion, 11, 909–918.
Simpson, C. F., and Kling, J. M. (1968) The mechanism of mitochondrial extrusion from phenylhydrazine-induced reticulocytes in the circulating blood, J. Cell Biol., 36, 103–109.
Nakajima, A., Kurihara, H., Yagita, H., Okumura, K., and Nakano, H. (2008) Mitochondrial extrusion through the cytoplasmic vacuoles during cell death, J. Biol. Chem., 283, 24128–24135.
Lyamzaev, K. G., Nepryakhina, O. K., Saprunova, V. B., Bakeeva, L. E., Pletjushkina, O. Y., Chernyak, B. V., and Skulachev, V. P. (2008) Novel mechanism of elimination of malfunctioning mitochondria (mitoptosis): formation of mitoptotic bodies and extrusion of mitochondrial material from the cell, Biochim. Biophys. Acta, 1777, 817–825.
Spees, J. L., Olson, S. D., Whitney, M. J., and Prockop, D. J. (2006) Mitochondrial transfer between cells can rescue aerobic respiration, Proc. Natl. Acad. Sci. USA, 103, 1283–1288.
Yasuda, K., Park, H. C., Ratliff, B., Addabbo, F., Hatzopoulos, A. K., Chander, P., and Goligorsky, M. S. (2010) Adriamycin nephropathy: a failure of endothelial progenitor cell-induced repair, Am. J. Pathol., 176, 1685–1695.
Cho, Y. M., Kim, J. H., Kim, M., Park, S. J., Koh, S. H., Ahn, H. S., Kang, G. H., Lee, J. B., Park, K. S., and Lee, H. K. (2012) Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations, PLoS One, 7, e32778.
Li, X., Zhang, Y., Yeung, S. C., Liang, Y., Liang, X., Ding, Y., Ip, M. S., Tse, H. F., Mak, J. C., and Lian, Q. (2014) Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage, Am. J. Respir. Cell Mol. Biol., 51, 455–465.
Otsu, K., Das, S., Houser, S. D., Quadri, S. K., Bhattacharya, S., and Bhattacharya, J. (2009) Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells, Blood, 113, 4197–4205.
Vallabhaneni, K. C., Haller, H., and Dumler, I. (2012) Vascular smooth muscle cells initiate proliferation of mesenchymal stem cells by mitochondrial transfer via tunneling nanotubes, Stem Cells Dev., 21, 3104–3113.
Acquistapace, A., Bru, T., Lesault, P. F., Figeac, F., Coudert, A. E., le Coz, O., Christov, C., Baudin, X., Auber, F., Yiou, R., Dubois-Rande, J. L., and Rodriguez, A. M. (2011) Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer, Stem Cells, 29, 812–824.
Mishra, P., and Chan, D. C. (2014) Mitochondrial dynamics and inheritance during cell division, development and disease, Nature Rev. Mol. Cell Biol., 15, 634–646.
Fontaine, K. M., Cooley, J. R., and Simon, C. (2007) Evidence for paternal leakage in hybrid periodical cicadas (Hemiptera: Magicicada spp.), PLoS One, 2, e892.
Dokianakis, E., and Ladoukakis, E. D. (2014) Different degree of paternal mtDNA leakage between male and female progeny in interspecific Drosophila crosses, Ecol. Evol., 4, 2633–2641.
Song, W. H., Ballard, J. W., Yi, Y. J., and Sutovsky, P. (2014) Regulation of mitochondrial genome inheritance by autophagy and ubiquitin-proteasome system: implications for health, fitness, and fertility, Biomed. Res. Int., 2014, 981867; DOI: 10.1155/2014/981867.
Sutovsky, P., Moreno, R. D., Ramalho-Santos, J., Dominko, T., Simerly, C., and Schatten, G. (1999) Ubiquitin tag for sperm mitochondria, Nature, 402, 371–372.
Sutovsky, P., Moreno, R. D., Ramalho-Santos, J., Dominko, T., Simerly, C., and Schatten, G. (2000) Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos, Biol. Reprod., 63, 582–590.
Sutovsky, P., Moreno, R., Ramalho-Santos, J., Dominko, T., Thompson, W. E., and Schatten, G. (2001) A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis, J. Cell Sci., 114, 1665–1675.
Sutovsky, P., Navara, C. S., and Schatten, G. (1996) Fate of the sperm mitochondria, and the incorporation, conversion, and disassembly of the sperm tail structures during bovine fertilization, Biol. Reprod., 55, 1195–1205.
Sutovsky, P., McCauley, T. C., Sutovsky, M., and Day, B. N. (2003) Early degradation of paternal mitochondria in domestic pig (Sus scrofa) is prevented by selective proteasomal inhibitors lactacystin and MG132, Biol. Reprod., 68, 1793–1800.
Shitara, H., Kaneda, H., Sato, A., Inoue, K., Ogura, A., Yonekawa, H., and Hayashi, J. I. (2000) Selective and continuous elimination of mitochondria microinjected into mouse eggs from spermatids, but not from liver cells, occurs throughout embryogenesis, Genetics, 156, 1277–1284.
Zorov, D. B., Isaev, N. K., Plotnikov, E. Y., Silachev, D. N., Zorova, L. D., Pevszner, I. B., Morosanova, M. A., Jankauskas, S. S., Zorov, S. D., and Babenko, B. A. (2013) Perspectives of mitochondrial medicine, Biochemistry (Moscow), 78, 979–990.
Skulachev, M. V., Antonenko, Y. N., Anisimov, V. N., Chernyak, B. V., Cherepanov, D. A., Chistyakov, V. A., Egorov, M. V., Kolosova, N. G., Korshunova, G. A., Lyamzaev, K. G., Plotnikov, E. Y., Roginsky, V. A., Savchenko, A. Y., Severina, I. I., Severin, F. F., Shkurat, T. P., Tashlitsky, V. N., Shidlovsky, K. M., Vyssokikh, M. Y., Zamyatnin, A. A., Jr., Zorov, D. B., and Skulachev, V. P. (2011) Mitochondrial-targeted plastoquinone derivatives. Effect on senescence and acute age-related pathologies, Curr. Drug Targets, 12, 800–826.
Smith, R. A., and Murphy, M. P. (2011) Mitochondria-targeted antioxidants as therapies, Discov. Med., 11, 106–114.
Takeda, K., Tasai, M., Akagi, S., Matsukawa, K., Takahashi, S., Iwamoto, M., Srirattana, K., Onishi, A., Tagami, T., Nirasawa, K., Hanada, H., and Pinkert, C. A. (2010) Microinjection of serum-starved mitochondria derived from somatic cells affects parthenogenetic development of bovine and murine oocytes, Mitochondrion, 10, 137–142.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E. Y. Plotnikov, V. A. Babenko, D. N. Silachev, L. D. Zorova, T. G. Khryapenkova, E. S. Savchenko, I. B. Pevzner, D. B. Zorov, 2015, published in Biokhimiya, 2015, Vol. 80, No. 5, pp. 642–650.
Rights and permissions
About this article
Cite this article
Plotnikov, E.Y., Babenko, V.A., Silachev, D.N. et al. Intercellular transfer of mitochondria. Biochemistry Moscow 80, 542–548 (2015). https://doi.org/10.1134/S0006297915050041
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297915050041