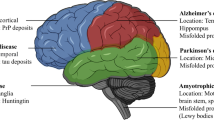
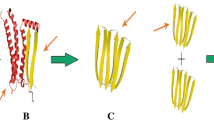
Abstract
A number of neurodegenerative disorders were recently coalesced into a group of proteinopathies based on the similarity of molecular mechanisms underlying their pathogenesis. A key step in the development of proteinopathies is a structural change that triggers aggregation of the proteins that are intrinsically prone to form aggregates owing to their physical and chemical properties. The review considers the recent progress in the field of proteinopathies with a special focus on the properties of aggregation-prone proteins, the main stages of the development of molecular pathology, and the role of cell clearance systems in the progression of neurodegeneration. Recent modifications made to the nomenclature of neurodegenerative diseases on the basis of the molecular mechanism of neurodegeneration are also addressed.
Similar content being viewed by others
Abbreviations
- NDD:
-
neurodegenerative disorder
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- UPS:
-
ubiquitin-proteasomal system
- APP:
-
β-amyloid precursor protein
References
Cummings J.L. 2003. Toward a molecular neuropsychiatry of neurodegenerative diseases. Ann. Neurol. 54, 147–154.
Jellinger K.A. 2010. Basic mechanisms of neurodegeneration: A critical update. J. Cell Mol. Med. 14, 57–87.
Skovronsky D.M., Lee V.M., Trojanowski J.Q. 2006. Neurodegenerative diseases: New concepts of pathogenesis and their therapeutic implications. Annu. Rev. Pathol. 1, 151–170.
Kurz A., Perneczky R. 2009. Neurobiology of cognitive disorders. Curr. Opin. Psychiatry. 22, 546–551.
Bekris L.M., Mata I.F., Zabetian C.P. 2010. The genetics of Parkinson disease. J. Geriatr. Psychiatry Neurol. 23, 228–242.
Bertram L., Tanzi R.E. 2008. Thirty years of Alzheimer’s disease genetics: The implications of systematic meta-analyses. Nature Rev. Neurosci. 9, 768–778.
See T.M., LaMarre A.K., Lee S.E., Miller B.L. 2010. Genetic causes of frontotemporal degeneration. J. Geriatr. Psychiatry Neurol. 23, 260–268.
Santpere G., Ferrer I. 2009. LRRK2 and neurodegeneration. Acta Neuropathol. 117, 227–246.
Goedert M., Jakes R. 2005. Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta. 1739, 240–250.
Woodruff-Pak D.S. 2008. Animal models of Alzheimer’s disease: therapeutic implications. J. Alzheimer’s Dis. 15, 507–521.
Bugos O., Bhide M., Zilka N. 2009. Beyond the rat models of human neurodegenerative disorders. Cell Mol. Neurobiol. 29, 859–869.
Ferrante R.J. 2009. Mouse models of Huntington’s disease and methodological considerations for therapeutic trials. Biochim. Biophys. Acta. 1792, 506–520.
Garringer H.J., Murrell J., D’Adamio L., Ghetti B., Vidal R. 2010. Modeling familial British and Danish dementia. Brain Struct. Funct. 214, 235–244.
Klein R.L., Wang D.B., King M.A. 2009. Versatile somatic gene transfer for modeling neurodegenerative diseases. Neurotox. Res. 16, 329–342.
Ninkina N.N., Ustyugov A.A., Buchman V.L. 2008. Modeling synucleinopathies in genetically modified animals: Successes and failures. Mol. Biol. (Moscow). 42, 747–761.
Buchman V.L., Ninkina N. 2008. Modulation of alpha-synuclein expression in transgenic animals for modelling synucleinopathies: Is the juice worth the squeeze? Neurotox. Res. 14, 329–341.
Telling G.C. 2008. Transgenic mouse models of prion diseases. Methods Mol. Biol. 459, 249–263.
Murphy R., Tsai A. 2006. Misbehaving Proteins: Protein (Mis)Folding, Aggregation, and Stability. Springer.
Tsvetkov P.O., Kulikova A.A., Golovin A.V., et al. 2010. Minimal Zn(2+) binding site of amyloid-β. Biophys. J. 99, L84–L86.
Ingelsson M., Hyman B.T. 2002. Disordered proteins in dementia. Ann. Med. 34, 259–271.
Maltsev A.V., Galzitskaya O.V. 2010. Formation and participation of nano-amyloids in pathogenesis of Alzheimer’s disease and other amyloidogenic diseases. Biomed. Khim. 56, 624–638.
Singleton A.B., Farrer M., Johnson J., et al. 2003. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 302, 841.
Citron M., Westaway D., Xia W., et al. 1997. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid-protein in both transfected cells and transgenic mice. Nature Med. 3, 67–72.
Cobb N.J., Surewicz W.K. 2009. Prion diseases and their biochemical mechanisms. Biochemistry. 48, 2574–2585.
Angot E., Steiner J.A., Hansen C., et al. 2010. Are synucleinopathies prion-like disorders? Lancet Neurol. 9, 1128–1138.
Cushman M., Johnson B. S., King O.D., et al. 2010. Prion-like disorders: Blurring the divide between transmissibility and infectivity. J. Cell Sci. 123, 1191–1201.
Jellinger K.A. 2003. General aspects of neurodegeneration. J. Neural. Transm. Suppl. 65, 101–144.
Uversky V.N. 2008. Alpha-synuclein misfolding and neurodegenerative diseases. Curr. Protein Pept. Sci. 9, 507–540.
Adlard P.A., Bush A.I. 2006. Metals and Alzheimer’s disease. J. Alzheimer’s Dis. 10, 145–163.
Zakharova E.I., Storozheva Z.I., Dudchenko A.M., Kubatiev A.A. 2010. Chronic cerebral ischaemia forms new cholinergic mechanisms of learning and memory. Int. J. Alzheimer’s Dis. 20. doi: 10.4061/2010/954589.
Shcherbatykh I., Carpenter D.O. 2007. The role of metals in the etiology of Alzheimer’s disease. J. Alzheimer’s Dis. 11, 191–205.
Kozin S.A., Zirah S., Rebuffat S., et al. 2001. Zinc binding to Alzheimer’s Abeta(1–16) peptide results in stable soluble complex. Biochem. Biophys. Res. Commun. 285, 959–964.
Kozin S.A., Mezentsev Y.V., Kulikova A.A., et al. 2011. Zinc-induced dimerization of the amyloid-β metal-binding domain 1-16 is mediated by residues 11-14. Mol. Biosyst. 7, 1053–1055.
Atwood C.S., Martins R.N., Smith M.A., Perry G. 2002. Senile plaque composition and posttranslational modification of amyloid-beta peptide and associated proteins. Peptides. 23, 1343–1350.
Oueslati A., Fournier M., Lashuel H.A. 2010. Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: Implications for Parkinson’s disease pathogenesis and therapies. Prog. Brain Res. 183, 115–145.
Martin L., Latypova X., Terro F. 2011. Post-translational modifications of tau protein: Implications for Alzheimer’s disease. Neurochem. Int. 58, 458–471.
Zirah S., Kozin S.A., Mazur A.K., et al. 2006. Structural changes of region 1-16 of the Alzheimer disease amyloid beta-peptide upon zinc binding and in vitro aging. J. Biol. Chem. 281, 2151–2161.
Tsvetkov P.O., Popov I.A., Nikolaev E.N., et al. 2008. Isomerization of the Asp7 residue results in zinc-induced oligomerization of Alzheimer’s disease amyloid beta(1-16) peptide. ChemBioChem. 9, 1564–1567.
Saha A.R., Ninkina N.N., Hanger D.P., et al. 2000. Induction of neuronal death by alpha-synuclein. Eur. J. Neurosci. 12, 3073–3077.
Caughey B., Lansbury P.T. 2003. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci. 26, 267–298.
Stefanova N., Reindl M., Neumann M., et al. 2005. Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am. J. Pathol. 166, 869–876.
Behl C., Davis J.B., Lesley R., Schubert D. 1994. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 77, 817–827.
Tanaka Y., Engelender S., Igarashi S., et al. 2001. Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum. Mol. Genet. 10, 919–926.
Kayed R., Head E., Thompson J.L., et al. 2003. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 300, 486–489.
Kabashi E., Valdmanis P.N., Dion P., et al. 2008. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nature Genet. 40, 572–574.
Vance C., Rogelj B., Hortobagyi T., et al. 2009. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 323, 1208–1211.
Kwiatkowski T.J., Bosco D.A., Leclerc A.L., et al. 2009. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 323, 1205–1208.
Mackenzie I.R., Neumann M., Bigio E.H., et al. 2009. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: Consensus recommendations. Acta Neuropathol. 117, 15–18.
Hicks G.G., Singh N., Nashabi A., et al. 2000. Fus deficiency in mice results in defective B-lymphocyte development and activation, high levels of chromosomal instability and perinatal death. Nature Genet. 24(2), 175–179.
Cook C., Zhang Y.J., Xu Y.F., et al. 2008. TDP-43 in neurodegenerative disorders. Exp. Opin. Biol. Ther. 8(7), 969–978.
Iguchi Y., Katsuno M., Niwa J., et al. 2009. TDP-43 depletion induces cell damage through dysregulation of Rho family GTPases. J. Biol. Chem. 284(33), 22059–22066.
Ninkina N., Papachroni K., Robertson D.C., et al. 2003. Neurons expressing the highest levels of gammasynuclein are unaffected by targeted inactivation of the gene. Mol. Cell Biol. 23, 8233–8245.
Sailer A., Büeler H., Fischer M., et al. 1994. No propagation of prions in mice devoid of PrP. Cell. 77(7), 967–968.
Anwar S., Peters O., Millership S., et al. 2011. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J. Neurosci. 31, 7264–7274.
Weyer S.W., Klevanski M., Delekate A., et al. 2011. APP and APLP2 are essential at PNS and CNS synapses for transmission, spatial learning and LTP. EMBO J. 30(11), 2266–2280.
Senior S.L. Ninkina N., Deacon R., et al. 2008. Increased striatal dopamine release and hyperdopam-inergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur. J. Neurosci. 27, 947–957.
Baka I.D., Ninkina N.N., Pinon L.G., et al. 1996. Intracellular compartmentalization of two differentially spliced s-rex/NSP mRNAs in neurons. Mol. Cell Neurosci. 7, 289–303.
Greten-Harrison B., Polydoro M., Tomita M.M., et al. 2010. αβΓ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc. Natl. Acad. Sci. U. S. A. 107, 19573–19578.
Buchman V.L, Adu J., Pinon L.G.P., et al. 1998. Persyn, a member of the synuclein family, influences neurofilament network integrity. Nature Neurosci. 1, 101–103.
Nguyen J.V., Soto I., Kim K.Y., et al. 2011. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc. Natl. Acad. Sci. U. S. A. 108, 1176–1181.
Fändrich M. 2007. On the structural definition of amyloid fibrils and other polypeptide aggregates. Cell Mol. Life Sci. 64, 2066–2078.
Sipe J.D, Benson M.D., Buxbaum J.N., et al. 2010. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 17(3–4), 101–104.
Lührs T., Ritter C., Adrian M., et al. 2005. 3D structure of Alzheimer’s amyloid-beta(1-42) fibrils. Proc. Natl. Acad. Sci. U. S. A. 102, 17342–17347.
Petkova A.T., Ishii Y., Balbach J.J., et al. 2002. A structural model for Alzheimer’s beta-amyloid fibrils based on experimental constraints from solid state NMR. Proc. Natl. Acad. Sci. U. S. A. 99, 16742–16747.
Iwata K., Fujiwara T., Matsuki Y., et al. 2006. 3D structure of amyloid protofilaments of beta2-micro-globulin fragment probed by solid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 103, 18119–18124.
Shewmaker F., Wickner R.B., Tycko R. 2006. Amyloid of the prion domain of Sup35p has an in-register parallel beta-sheet structure. Proc. Natl. Acad. Sci. U. S. A. 103, 19754–19759.
Nelson R., Sawaya M. R., Balbirnie M., et al. 2005. Structure of the cross-beta spine of amyloid-like fibrils. Nature. 435, 773–778.
Chiti F., Dobson C.M. 2006. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366.
Tycko R. 2011. Solid-state NMR studies of amyloid fibril structure. Annu. Rev. Phys. Chem. 62, 279–299.
Guijarro J.I., Sunde M., Jones J.A., et al. 1998. Amyloid fibril formation by an SH3 domain. Proc. Natl. Acad. Sci. U. S. A. 95, 4224–4228.
Fowler D.M., Koulov A.V., Alory-Jost C., et al. 2006. Functional amyloid formation within mammalian tissue. PLoS Biol. 4, e6.
Saupe S.J. 2007. A short history of small s, a prion of the fugus Podospora anserine. Prion. 1, 110–115.
Wang X., Hammer N.D., Chapman M.R. 2008. The molecular basis of functional bacterial amyloid polymerization and nucleation. J. Biol. Chem. 283, 21530–21539.
Bemporad F., Calloni G., Campioni S., et al. 2006. Sequence and structural determinants of amyloid fibril formation. Acc. Chem. Res. 39, 620–627.
Heise H., Hoyer W., Becker S., et al. 2005. Molecularlevel secondary structure, polymorphism, and dynamics of full-length α-synuclein fibrils studied by solid state NMR. Proc. Natl. Acad. Sci. U. S. A. 102, 15871–15876.
Madine J., Copland A., Serpell L. C., Middleton D. A. 2009. Cross-beta spine architecture of fibrils formed by the amyloidogenic segment NFGSVQFV of medin from solid-state NMR and X-ray fiber diffraction measurements. Biochemistry. 48, 3089–3099.
Luca S., Yau W. M., Leapman R., Tycko R. 2007. Peptide conformation and supramolecular organization in amylin fibrils: Constraints from solid state NMR. Biochemistry. 46, 13505–13522.
Paravastu A.K., Petkova A.T., Tycko R. 2006. Polymorphic fibril formation by residues 10–40 of the Alzheimer’s β-amyloid peptide. Biophys. J. 90, 4618–4629.
Verel R., Tomka I.T., Bertozzi C., et al. 2008. Polymorphism in an amyloidlike fibril-forming model peptide. Angew. Chem. Int. Ed. Engl. 47, 5842–5845.
Petkova A.T., Leapman R.D., Guo Z.H., et al. 2005. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science. 307, 262–265.
Sawaya M.R., Sambashivan S., Nelson R., et al. 2007. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature. 447, 453–457.
Lesné S., Koh M.T., Kotilinek L., et al. 2006. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 440, 352–357.
Shankar G.M., Li S., Mehta T.H., et al. 2008. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature Med. 14, 837–842.
Hardy J., Selkoe D.J. 2002. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 297, 353–356.
Paravastu A.K., Qahwash I., Leapman R.D., et al. 2009. Seeded growth of β-amyloid fibrils from Alzheimer’s brain-derived fibrils produces a distinct fibril structure. Proc. Natl. Acad. Sci. U. S. A. 106, 7443–7448.
Geser F., Martinez-Lage M., Kwong L.K., et al. 2009. Amyotrophic lateral sclerosis, frontotemporal dementia and beyond: The TDP-43 diseases. J. Neurol. 256, 1205–1214.
Galpern W.R., Lang A.E. 2006. Interface between tauopathies and synucleinopathies: A tale of two proteins. Ann. Neurol. 59, 449–458.
Alafuzoff I., Parkkinen L., Al-Sarraj S., et al. 2008. Assessment of alpha-synuclein pathology: A study of the BrainNet Europe Consortium. J. Neuropathol. Exp. Neurol. 67, 125–143.
Kovacs G.G., Alafuzoff I., Al-Sarraj S., et al. 2008. Mixed brain pathologies in dementia: The BrainNet Europe consortium experience. Dement. Geriatr. Cogn. Disord. 26, 343–350.
Dohm C.P., Kermer P., Bähr M. 2008. AAggregopathy in neurodegenerative diseases: Mechanisms and therapeutic implication. Neurodegener. Dis. 5, 321–338.
Bossy-Wetzel E., Schwarzenbacher R., Lipton S.A. 2004. Molecular pathways to neurodegeneration. Nature Med. Suppl. 10, S2–S9.
Su H., Wang X. 2010. The ubiquitin-proteasome system in cardiac proteinopathy: A quality control perspective. Cardiovasc. Res. 85, 253–262.
Guo P.C., Zhou Y.Y., Ma X.X., Li W.F. 2010. Structure of Hsp33/YOR391Cp from the yeast Saccharomyces cerevisiae. Acta Crystallogr. Sect. F. Struct. Biol. Cryst. Commun. 66, 1557–1561.
Chai Y., Koppenhafer S.L., Bonini N.M., Paulson H.L. 1999. Analysis of the role of heat shock protein (Hsp) molecular chaperones in polyglutamine disease. J. Neurosci. 19, 10338–10347.
McNaught K.S., Shashidharan P., Perl D.P., et al. 2002. Aggresome-related biogenesis of Lewy bodies. Eur. J. Neurosci. 16, 2136–2148.
Wacker J.L., Huang S.Y., Steele A.D., et al. 2009. Loss of Hsp70 exacerbates pathogenesis but not levels of fibrillar aggregates in a mouse model of Huntington’s disease. J. Neurosci. 29, 9104–9114.
Lehman N.L. 2009. The ubiquitin proteasome system in neuropathology. Acta Neuropathol. 118, 329–347.
Shadrina M.I., Semenova E.V., Slominsky P.A., et al. 2007. Effective quantitative real-time polymerase chain reaction analysis of the parkin gene (PARK2) exon 1-12 dosage. BMC Med. Genet. 8, 6.
Hardy J., Lewis P., Revesz T., et al. 2009. The genetics of Parkinson’s syndromes: A critical review. Curr. Opin. Genet. Dev. 19, 254–265.
Bedford L., Hay D., Devoy A., et al. 2008. Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J. Neurosci. 28, 8189–8198.
Komatsu M., Waguri S., Chiba T., et al. 2006. Loss of autophagy in the central nervous system causes neuro-degeneration in mice. Nature. 441, 880–884.
Pickford F., Masliah E., Britschgi M., et al. 2008. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J. Clin. Invest. 118, 2190–2199.
Hara T., Nakamura K., Matsui M., et al. 2006. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 441, 885–889.
Mijaljica D., Prescott M., Devenish R.J. 2007. Different fates of mitochondria: Alternative ways for degradation? Autophagy. 3, 4–9.
Rusten T.E., Filimonenko M., Rodahl L.M., et al. 2008. ESCRTing autophagic clearance of aggregating proteins. Autophagy. 4(2), 233–236.
Lee S.J., Kim S.J., Kim I.K., et al. 2003. Crystal structures of human DJ-1 and Escherichia coli Hsp31, which share an evolutionarily conserved domain. J. Biol. Chem. 278, 44552–44559.
Braak H., Alafuzoff I., Arzberger T., et al. 2006. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112, 389–404.
Ninkina N., Peters O., Millership S., et al. 2009. Gamma-synucleinopathy: Neurodegeneration associated with overexpression of the mouse protein. Hum. Mol. Genet. 18, 1779–1794.
Goedert M., Clavaguera F., Tolnay M. 2010. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 33, 317–325.
Khachaturian Z.S., Snyder P.J., Doody R. 2009. A roadmap for the prevention of dementia II: Leon Thal Symposium 2008. Alzheimer’s Dement. 5, 85–92.
Smith C.U. 2009. Chapter 24. The coming of molecular biology and its impact on clinical neurology. Handb. Clin. Neurol. 95, 361–372.
Bachurin S.O., Ustyugov A.A., Peters O., Shelkovnikova T.A., Buchman V.L., Ninkina N.N. 2009. Hindering of proteinopathy-induced neurodegeneration as a new mechanism of action for neuroprotectors and cognition enhancing compounds. Doklady Biochem. Biophys. 428, 235–238.
Yamashita M., Nonaka T., Arai T., et al. 2009. Methylene blue and dimebon inhibit aggregation of TDP-43 in cellular models. FEBS Lett. 583, 2419–2424.
Menzies F.M., Rubinsztein D.C. 2010. Broadening the therapeutic scope for rapamycin treatment. Autophagy. 6, 286–287.
Gervais F., Paquette J., Morissette C., et al. 2007. Targeting soluble Aβ peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol. Aging. 28, 537–547.
Shemesh O.A., Spira M.E. 2011. Rescue of neurons from undergoing hallmark tau-induced Alzheimer’s disease cell pathologies by the antimitotic drug paclitaxel. Neurobiol. Dis. 43, 163–175.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.A. Shelkovnikova, A.A. Kulikova, Ph.O. Tsvetkov, O. Peters, S.O. Bachurin, V.L. Buchman, N.N. Ninkina, 2012, published in Molekulyarnaya Biologiya, 2012, Vol. 46, No. 3, pp. 402–415.
Rights and permissions
About this article
Cite this article
Shelkovnikova, T.A., Kulikova, A.A., Tsvetkov, P.O. et al. Proteinopathies, neurodegenerative disorders with protein aggregation-based pathology. Mol Biol 46, 362–374 (2012). https://doi.org/10.1134/S0026893312020161
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893312020161