-
PDF
- Split View
-
Views
-
Cite
Cite
Alette A.M. Langenhoff, Ivonne Nijenhuis, Nico C.G. Tan, Maria Briglia, Alexander J.B. Zehnder, Gosse Schraa, Characterisation of a manganese-reducing, toluene-degrading enrichment culture, FEMS Microbiology Ecology, Volume 24, Issue 2, October 1997, Pages 113–125, https://doi.org/10.1111/j.1574-6941.1997.tb00428.x
Close - Share Icon Share
Abstract
A bacterial culture (LET-13) was enriched, which uses toluene as sole carbon and energy source, and manganese oxide as terminal electron acceptor. The culture is able to degrade a variety of substituted monoaromatic compounds like p-hydroxy-benzylalcohol, p-hydroxy-benzaldehyde, p-hydroxy-benzoate, phenol and the three isomers of cresol. Benzene, ethylbenzene, all xylenes and naphthalene were not degraded under the experimental conditions used. Based on the results of growth experiments and the detection of intermediates, it is concluded that toluene is degraded via a methyl hydroxylation. A possible side reaction can lead to the formation of cresol. The organisms in the culture look similar; motile rods, which are Gram-negative, oxidase-negative and catalase-negative. The culture was partly identified by phylogenetic analysis of cloned rDNA sequences. The phylogenetic analysis showed that at least two major groups of bacteria are present. One group of bacteria shows 70–80% similarity (based on 16S rRNA gene sequence data) with the Bacteroides-Cytophaga group, and one group consists of members of the β-subclass of the Proteobacteria.
1 Introduction
Toluene is one of the nonoxygenated, monoaromatic compounds that are present in petroleum (others are, e.g., benzene, ethylbenzene and xylenes). Due to leakages of petroleum storage tanks and spills at petroleum wells, these hydrocarbons contribute significantly to groundwater contamination. In anaerobic environments, they often appear to accumulate. Toluene is also known to be formed biologically [1] in anoxic freshwater sediments.
Most aromatic hydrocarbons are rapidly degraded under aerobic conditions; oxygen is involved in activation and fission of the aromatic ring and is the terminal electron acceptor. Information on the anaerobic microbial degradation of these compounds has been emerging in the literature only in the last decade. Recently, we have published data about the bacterial degradation of toluene under manganese-reducing conditions [2]. Such a degradation has never been demonstrated before. Several manganese-reducing bacteria have been described in the literature, but none of them can degrade aromatic compounds [3,4]. The manganese-reducing bacterium Shewanella putrefaciens MR-1 obtains energy for growth by using H2, formate, or lactate as electron donor. The bacterium has been classified as a member of the γ-subclass of the Proteobacteria[5]. Geobacter metallireducens degrades compounds like acetate, butyrate, propionate and ethanol with amorphous manganese oxide as electron acceptor. G. metallireducens can degrade toluene with iron oxide as electron acceptor, but not with manganese oxide as electron acceptor (unpublished results). G. metallireducens has been categorized in the δ-subclass of the Proteobacteria and is closely related to Desulfuromonas acetoxidans[6].
Toluene has been found anaerobically degradable under denitrifying [7–19], iron-reducing [20], sulfate-reducing [21–25] and methanogenic conditions [26–29]. Various bacteria have been isolated and parts of degradation routes have been elucidated. An overview of the anaerobic toluene metabolism is given by Frazer et al. [30].
Studies on the formation of intermediates in denitrifying bacteria [8,16,17], the sulfate-reducing bacterium Tol2 [25] and the iron-reducing bacterium G. metallireducens (formerly known as GS-15) [20], suggest that the degradation proceeds through oxidation of the methyl group. Benzoyl-CoA is formed via benzylalcohol, benzaldehyde and benzoate, upon which ring fission occurs. Another pathway has been found for the denitrifying strain T1 [11] and in a sulfate-reducing enrichment culture [21]. Toluene is first converted with acetyl-CoA to phenylpropionyl-CoA, which is further degraded to benzoyl-CoA. About 15% of the toluene would react with succinyl-CoA to form two dead-end products: benzylsuccinate and benzylfumarate. The same dead-end products have been detected with the sulfate-reducing bacterium strain PRTOL1 [22], but the main degradation pathway of toluene has not been clarified. Recently, the degradation of toluene in the denitrifying bacterium Thauera aromatica has been demonstrated to start with an initial addition of fumarate to the methyl group of toluene, to yield benzylsuccinate [31]. A further degradation of benzylsuccinate to benzoyl-CoA is suggested to occur via β-oxidation. Alternative pathways via a ring hydroxylation to cresol or a carboxylation to toluate have also been discussed, but no experimental data exist to support the occurrence of such a pathway [7,16,20]. Small amounts of p-cresol have been detected in a methanogenic enrichment culture with toluene, in which they seem to be formed via the oxidation of the aromatic ring [28]. It was demonstrated that the hydroxyl group originated from water. However, the conversion of toluene to p-cresol is very slow, compared with the rapid breakdown of toluene in this culture.
Here we report the physiological and phylogenetical characterization of an anaerobic enrichment culture that mineralizes toluene in the presence of amorphous manganese oxide as electron acceptor. The culture has been maintained with toluene as sole carbon and energy source and with manganese oxide as electron acceptor for more than 2 years. Data on the use of alternative electron acceptors, the growth on possible intermediates and the identification of formed intermediates are discussed in this paper.
2 Materials and methods
2.1 Chemicals
All aromatic compounds were purchased from E. Merck (Darmstadt, Germany) and were of analytical grade. [13C]toluene was purchased from ISOTEC (Miamisburg, OH, USA) and 99.3% was α-labelled.
2.2 Enrichment and cultivation
The enrichment culture had been obtained from sediment columns in which toluene was transformed under manganese-reducing conditions [2]. Routine cultivations were carried out in 115-ml serum bottles with 20 ml of anaerobic medium and amorphous manganese oxide (δMnO2) as electron acceptor, as described by Langenhoff et al. [2]. In addition, 2 mM of nitrilotriacetic acid (NTA) was added to the medium to solubilize Mn(IV). Toluene was added from a heat sterilized water-saturated solution (2 ml), to obtain a medium concentration of 300 μM (18 μmol/bottle). Higher concentrations were not used because of inhibitory effects. Toluene was re-added when depleted. We have demonstrated in previous experiments that the addition of sterile Rhine river sediment was necessary to maintain the activity of the culture [2]. In further experiments, we found that a concentrated supernatant of the Rhine river sediment could be used as well. The supernatant was prepared by shaking 40 g of sterile Rhine river sediment and 40 ml of manganese-reducing medium anaerobically at 30°C overnight. This was followed by centrifugation and filter sterilization (0.22 μm Durapore filter, Millipore, USA). 2 ml of this Rhine river sediment supernatant was added to each of the batches. A gas phase of N2/CO2 (80%/20%), and a pressure of 1.3 bar were used. The bottles were incubated stationary in the dark at 30°C.
Transfer of the enrichment cultures (10% v/v) to bottles containing fresh medium was done after transformation of approximately 100 μmol of toluene. Transfers were normally made after 30–60 days, depending on the rate of transformation.
2.3 Purification
Attempts were made to further purify the manganese-reducing, toluene-degrading bacterial culture using dilution series in liquid cultures and on solid media. Anaerobic agar roll tubes [32] or anaerobic agar plates were made with anaerobic medium with and without manganese oxide, 2% highly purified noble agar (Difco, Detroit, MI, USA), and 0.3 mM toluene as substrate. The tubes or plates were inoculated with 0.1% (v/v) of the enrichment culture. The solidified agar in roll tubes or plates without manganese oxide was covered with a second agar layer in which amorphous manganese oxide was present. Both the tubes and the plates with one or a double layer of agar were incubated at 30°C in an anaerobic glovebox. The formation of clearing zones in the agar overlays (dark by the presence of manganese oxide) were used to indicate growth of manganese-reducing bacteria. This method has been described previously by Nealson [4], who found that manganese-reducing bacteria do not form visible colonies on solid media. Upon clearing of the agar, agar from the edges of these clearing zones was transferred to anaerobic manganese-reducing liquid medium.
2.4 Effect of temperature and pH
The temperature optimum for toluene degradation was determined in two-liquid-phase batches. A two-liquid-phase system of medium and hexadecane was used because of the low water solubility of toluene. Such a system creates a constant low toluene concentration in the medium and a constant flux of toluene from the organic phase to the aquatic medium. To 115-ml serum bottles with 20 ml of anaerobic manganese-reducing medium with NTA, 0.5 ml of hexadecane containing 0.2 M toluene was added. This resulted in a concentration in the liquid phase of 190 μM toluene [2]. The bottles were inoculated with 10% (v/v) of the toluene-degrading enrichment culture, and incubated at 4, 10, 20, 25, 30, 37, 43 and 55°C stationary in the dark.
The effect of pH on the toluene degradation rate was tested in similar two-liquid-phase batches. The pH was varied by applying different partial pressures of CO2 in the gas phase and varying the concentration of phosphate buffer (K2HPO4 and NaH2PO4·2H2O). It was confirmed that the pH at the end of each experiment was the same as the initial pH.
All experiments were performed in triplicate. Controls were made with 1 mM of sodium azide (an inhibitor of electron transport-linked respiration) and 0.175% of formaldehyde (a general inhibitor of biological activity). Both are known to inhibit manganese-reducing bacteria [33].
2.5 Electron microscopy
For negative staining, cells were fixed in 3% (v/v) glutaraldehyde and 2% (w/v) paraformaldehyde in 0.1 M of sodium phosphate buffer pH 7.2. A formvar-coated copper grid (150 mesh, ∅ 3 mm) was placed on a drop of cell suspension for 15 min. After washing the grid three times with distilled water, the grid was air-dried. After drying, the cells were contrasted with uranyl acetate and examined with a Jeol EM 1200 ex II transmission electron microscope.
2.6 Electron acceptor and donor utilization
Experiments were carried out in 115-ml serum bottles with 20 ml of anaerobic manganese-reducing medium with NTA, which had been inoculated with 10% (v/v) of the toluene-degrading enrichment culture. Thiosulfate (20 mM), fumarate (20 mM), nitrate (20 mM), and oxygen were tested as electron acceptor with toluene in hexadecane (0.2 M) as substrate. Controls were prepared with manganese oxide as an electron acceptor and without electron acceptor.
Various possible intermediates in the degradation of toluene and different monoaromatic compounds were tested for their degradability by the enrichment culture. Compounds tested in one-liquid-phase batches were benzylalcohol, benzoate, p-hydroxy-benzylalcohol, p-hydroxy-benzaldehyde, p-hydroxy-benzoate, o-, m- and p-toluate, and phenol (all at 100 μM). Toluene, benzaldehyde, o-, m- and p-cresol, o-, m- and p-xylene, benzene, ethylbenzene, styrene and naphthalene were tested in two-liquid-phase batches. A two-liquid-phase system was used because of the low water solubility of these compounds. 0.5 ml of hexadecane containing 0.2 M substrate was added. Controls were taken along to test for chemical interactions between the aromatic substrates and manganese oxide. They were incubated under similar conditions, but without inoculum added.
Intermediates in the degradation of toluene were measured in one-liquid-phase batches. In time, liquid samples were taken and analyzed with HPLC and GC-MS. Higher concentrations of intermediates were obtained by adding 1 mM fluoroacetate, an inhibitor of the tricarboxylic acid cycle. The inhibitor was added after 50 μmol of toluene was degraded [16].
Determination of intermediates was also done with [13C]toluene. Cultures that had degraded 50 μmol of [12C]toluene in a one-liquid-phase system, were fed [13C]toluene (99.3% was α-labelled). The formation of labelled intermediates was followed by GC-MS.
All experiments were performed at least in triplicate.
2.7 Isolation of nucleic acids
Nucleic acids were extracted from cultures that had degraded approximately 80 μmol of toluene. A 10-ml medium sample was centrifuged at 13 000 rpm for 20 minutes. The pellet was resuspended in 400 μl of autoclaved TE buffer (10 mM Tris/HCl, 1 mM EDTA, pH 8.0) and transferred to a 1.5 ml Eppendorf tube. 200 μl of Tris/HCl buffered phenol (pH 8.0) was added together with 300 μl of glass beads (∅ 0.11 mm). The cells were disrupted by beat-beating during 5 min, using a cell homogenizer MSK (Braun, Melsungen, Germany) under CO2-cooling, and subsequently centrifuged (15 min at 13 000 rpm). Nucleic acids were extracted from the aqueous phase with a phenol/chloroform/isoamylalcohol-mixture (25/24/1 (v/v/v)), and after centrifugation (15 min at 13 000 rpm), the aqueous phase was extracted by chloroform/isoamylalcohol (24/1 (v/v)). After centrifugation (15 min at 13 000 rpm), the nucleic acids were precipitated with 0.5 ml of isopropanol and 40 μl of 3 M sodium acetate (pH 5.2) at −70°C for 30 min. After centrifugation (15 min at 13 000 rpm), the nucleic acid pellet was washed with 70% ethanol, dried under vacuum and resuspended in 100 μl TE buffer. The quality of the extracts was analyzed by agarose gel electrophoresis, followed by ethidium bromide staining [34]. The DNA and rRNA extracts were used for dot blot hybridization, polymerase chain reaction (PCR), cloning, and temperature gradient gel electrophoresis (TGGE).
2.8 Dot blot hybridization
The sequences for the used 16S rRNA oligonucleotide probes and their target organisms are listed in Table 1. All oligonucleotides for dot blot hybridizations were synthesized by Pharmacia (Uppsala, Sweden) and were 5′ end labelled with [γ32P]ATP (3000 Ci/mmol, Amersham, Little Chalfont, UK) [35].
Summary of the oligonucleotide probes used in this study
| Probe | Target group | Sequence | Ref. |
| EUB338 | Bacteria | 5′-GCTGCCTCCCGTAGGAGT-3′ | [53] |
| ARC915 | Archaea | 5′-GTGCTCCCCCGCCAATTCCT-3′ | [54] |
| BET42a | β-subclass of Proteobacteria | 5′-GCCTTCCCACTTCGTTT-3′ | [48] |
| GAM42a | γ-subclass of Proteobacteria | 5′-GCCTTCCCACATCGTTT-3′ | [48] |
| SRB385 | sulfate-reducing bacteria | 5′-CGGCGTCGCTGCGTCAGG-3′ | [53] |
Summary of the oligonucleotide probes used in this study
| Probe | Target group | Sequence | Ref. |
| EUB338 | Bacteria | 5′-GCTGCCTCCCGTAGGAGT-3′ | [53] |
| ARC915 | Archaea | 5′-GTGCTCCCCCGCCAATTCCT-3′ | [54] |
| BET42a | β-subclass of Proteobacteria | 5′-GCCTTCCCACTTCGTTT-3′ | [48] |
| GAM42a | γ-subclass of Proteobacteria | 5′-GCCTTCCCACATCGTTT-3′ | [48] |
| SRB385 | sulfate-reducing bacteria | 5′-CGGCGTCGCTGCGTCAGG-3′ | [53] |
Dot blot hybridizations were performed on Hybond N+ filters (Amersham). Nucleic acid extracts of LET-13 were applied to the membrane with a Hybri.Dot manifold (Gibco BRL, Life Science Technologies, Gaithersburg, MD, USA) and immobilized by UV light (4 min). Escherichia coli (γ-Proteobacterium), Synthrophobacter wolinii (DSM 2805, sulfate-reducing bacterium) and Methanosaeta soehngenii (DSM 2139, Archaea) were used as positive controls. All membranes were pretreated with 10 ml of hybridization buffer (0.5 mM phosphate buffer, 7% sodium dodecyl sulfate (SDS), 1% bovine serum albumine, and 1 mM EDTA, pH 7.2) for 30 min. The probes were 5′-labelled with 32P by using [γ-32P]ATP and polynucleotide kinase. 1 μl (100 ng) of the probe was mixed with 2 μl of 10×kinase buffer [34], 1 μl of T4 polynucleotide kinase (Gibco BRL), 1.5 μl of [γ-32P]ATP (3000 mCi/mmol, Amersham), and water to obtain a total volume of 20 μl. This mixture was incubated at 37°C for 30 min. The membranes were hybridized overnight at a temperature of 46°C and rinsed with 10 ml of 1 mM EDTA and 5×SSC (0.15 M NaCl, 0.015 M sodium citrate, pH 7.0). Finally, the membranes were washed in 10 ml of 1% SDS, 1×SSC at 48°C, sealed in polyethylene foil, and exposed to a phosphor storage screen for 2 h. The screen was scanned for radioactive response on a Phosphor Imager (Molecular Dynamics, Sunnyvale, CA, USA). The digital signals were processed by the manufacturers software (ImageQuant).
2.9 PCR amplification, isolation, cloning and sequencing of the 16S rRNA gene
The 16S-targeted polymerase chain reaction (PCR) was used to amplify 16S rRNA genes (1.5 kb fragments) of the bacteria present in the enrichment culture. Two eubacterial universal primers were used: the forward primer corresponding to E. coli positions 8–27 (5′-CACGGATCCAGAGTTTGATC/T(A/C)TGGCTCAG) and the reverse primer corresponding to E. coli positions 1493–1510 (5′-GTGCTGCAGGGTTACCTTGTTACGACT). The PCR assay (100 μl) contained amplification buffer (20 mM Tris/HCl pH 8.4, 50 mM KCl), 3 mM MgCl2, 200 μM of each deoxynucleotide, 0.2 μM of each primer, 2.5 U Taq DNA polymerase (Life Technologies) and 1 μg template DNA. The PCR involved an initial denaturation step of 4 min at 90°C, followed by 35 amplification cycles: 94°C for 45 s, 54°C for 45 s, and 68°C for 2 min. This was followed by a final 7-min incubation at 68°C.
The isolation, cloning and sequence analyses manipulations were done using previously described procedures [34], with the following modifications. The amplification products were isolated and purified by agarose gel electrophoresis, excised from the gel and cleaned with glass milk (GlassMAX™, Gibco BRL). The fragments were cloned in a pGEMT vector (Promega, Madison, WI, USA), and plasmid preparation was done with the Wizard kit (Promega). The positive clones were sequenced by using the dideoxy chain termination reaction [36], using an automated DNA sequencer model 373A (Licon, USA). The resulting sequences were compared with the 16S rRNA sequences available in the literature and EMBL data bank using the FASTA program [37]. The sequences of clones I, II and III were deposited in the EMBL data bank under accession numbers Y13377, Y13202 and Y13812, respectively.
2.10 Temperature gradient gel electrophoresis (TGGE)
TGGE (Diagen TGGE system, Diagen GmbH, Düsseldorf, Germany) was used to obtain a band profile of the 16S rRNA fragments present in the DNA from the enrichment culture [38]. The 16S rRNA fragments of the V6–V8 regions (0.4 kb) originated from direct amplification of the DNA extracted from the enrichment culture, and from the whole 16S rRNA cloned fragments. The primers used were universal eubacterial primers: the forward U968-GC primer (5′-[GC-clamp]AACGCGAAGAACCTTAC-3′) and the reverse primer L1401 (5′-CGGTGTGTACAAGACCC-3′). The presence of the GC-clamp on one of the two primers is required to improve separation of the bands in the gel [39]. The PCR assay contained the reagents in the same proportions as described before. PCR conditions were 25 cycles (94°C for 20 s, 56°C for 20 s, and 68°C for 40 s). A volume of 6 μl of the PCR products was used for the TGGE run.
The TGGE consisted of polyacrylamide gel (6% (w/v) acrylamide, 0.1% (w/v) bis-acrylamide, 8 M urea, 20% (v/v) formamide, 2% (v/v) glycerol) and 1×TAE buffer [38]. A temperature range (37°C–46°C) at a constant voltage (120 V) for 15 h was chosen. After the electrophoresis, the band profile on the gel was visualized by silver staining the gel as follows: (a) 3 min fixing in 10% ethanol and 0.5% acetic acid; (b) 10 min staining in 10% ethanol and 0.5% acetic acid and 0.2% AgNO3; (c) 2–3 min washing in demineralized water; (d) developing in 1.5% NaOH, 0.1% formaldehyde and 0.01% NaBH4, untill visualization of the bands; (e) 5 min fixing in the same solution as in a; (f) 5 min washing in demineralized water; (g) 7 min treatment in a preservation solution containing 25% ethanol and 10% glycerol; (h) covering the gel with cellophane and drying overnight in the oven at 60°C.
2.11 Other methods
Gram staining, and oxidase and catalase reactivity tests were performed by standard procedures [40].
2.12 Analytical procedures
Toluene, benzene, ethylbenzene, styrene and o-, m- and p-xylene were measured gas chromatographically by headspace analysis [2].
Benzylalcohol, benzaldehyde, benzoate, o-, m- and p-cresol, phenol, p-hydroxy-benzylalcohol, p-hydroxy-benzaldehyde, p-hydroxy-benzoate, o-, m- and p-toluate, ethylbenzene and naphthalene were measured by high-performance liquid chromatography. Liquid samples (0.5 ml) were taken by syringe, centrifuged (13 000 rpm for 3 min) and analyzed on a HPLC (LKB, Bromma, Sweden). Samples (20 μl) were injected by using an autosampler (Spectra System AS1000) onto two Chromspher C8 columns (Chrompack, Bergen op Zoom, The Netherlands) connected in series at room temperature. The mobile phase was 20% acetonitrile and 80% 10 mM H2SO4 at a flow rate of 0.6 ml/min. The eluted compounds were identified and quantified with an UV detector (LKB 2158 Uvicord SD, Bromma, Sweden) at 206 nm.
To identify and quantify aromatic intermediates by GC-MS, the samples were extracted (1:1) overnight in chloroform. The chloroform extracts were analyzed on a Hewlett-Packard 5890 series II gas chromatograph (Hewlett Packard, Amsterdam, The Netherlands) equipped with a mass selective detector (HP 5971A series mass selector). The samples (1 μl) were injected on a fused silica analytical column (HP5, 30 m×0.25 mm) with an autosampler (HP, series 7673A). Oven temperature conditions for the separation were 40°C for 2 min, 10°C/min to 250°C, 250°C for 5 min. Data were acquired using either a full scan mode for identification of metabolites, or a selected ion monitoring mode (SIM) for quantification. The GC column did not allow the separation of m-cresol from p-cresol. Hence, when both compounds could be present, they were reported as summed parameter (m+p)-cresol.
3 Results
3.1 Enrichment
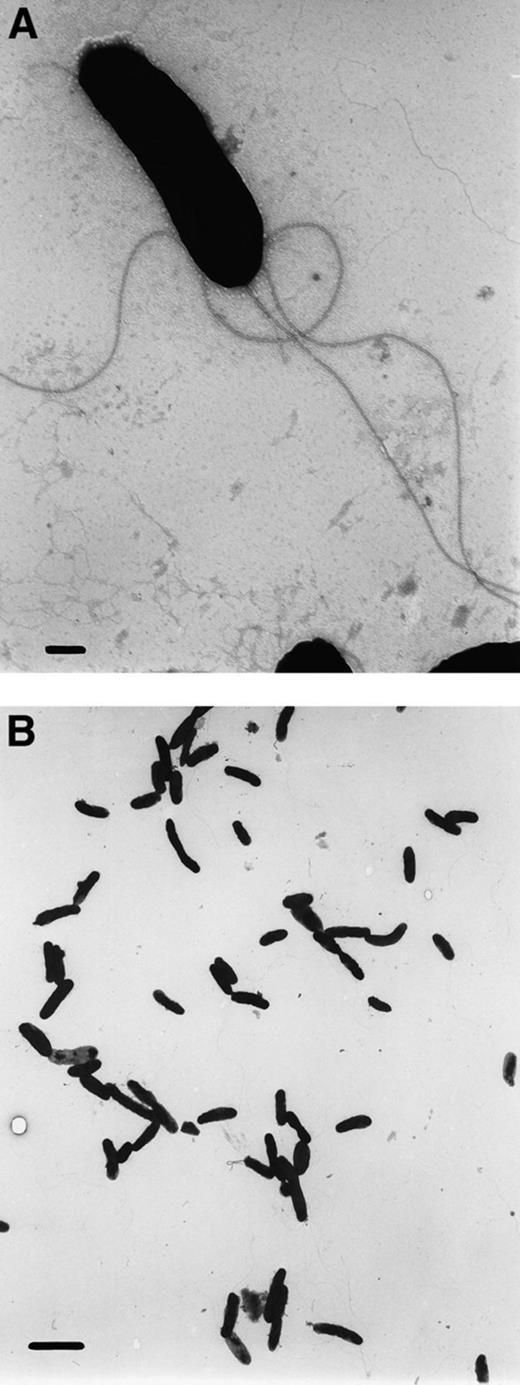
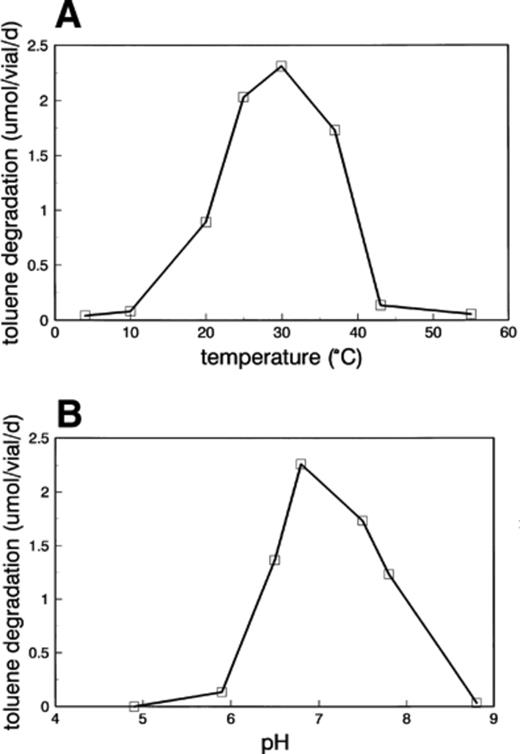
A microscopically stable enrichment culture, capable of mineralizing toluene in the presence of amorphous manganese oxide, has been obtained via repeated dilution series and transfers in liquid media after 2 years of cultivation. The culture, which will further be referred to as LET-13, consists mainly of gram-negative, motile rods that are oxidase- and catalase-negative, and microscopically identical. The rods are about 1–2 μm long, and possess several peritrichious flagellae (up to 6 μm in length), irregularly distributed around the cell (Fig. 1). LET-13 grows optimally between 25 and 35°C (Fig. 2A), whereas at 4 and 55°C no growth was observed. The toluene degradation rate was maximal around pH 7.0 (Fig. 2B). No degradation of toluene was observed below pH 4.9 and above pH 8.8.
Electron micrographs of culture LET-13; bars indicate 0.2 μm and 2 μm, respectively.
Temperature (A) and pH (B) dependence of the toluene degradation rate of LET-13 in two-liquid-phase incubations with amorphous manganese oxide as electron acceptor. The results of the triplicates were within 5% of each other.
Cultivation in agar roll tubes or on agar plates was not successful. Clearing zones, indicating the presence of manganese-reducing bacteria, did develop in the double-layer agar roll tubes and on the double-layer agar plates. However, after transferring the clearing zones into liquid medium, no degradation of toluene was observed, during 120 days of incubation.
3.2 Growth of LET-13 with alternative electron acceptors and donors
Nitrate and oxygen were both found to support growth of LET-13 with toluene as substrate. Transfer of these cultures into fresh medium with amorphous manganese oxide as electron acceptor resulted again in toluene degradation and manganese reduction. However, routine cultivation of LET-13 with nitrate or oxygen as electron acceptor and toluene as substrate, resulted after several transfers in enrichment cultures, which could no longer use manganese oxide as electron acceptor. No degradation of toluene was observed with fumarate or thiosulfate as electron acceptor during 150 days of incubation.
LET-13 is able to oxidize and grow on benzylalcohol, benzaldehyde, benzoate, phenol, p-hydroxy-benzylalcohol, p-hydroxy-benzaldehyde and p-hydroxy-benzoate in the presence of manganese oxide. All cultures could be transferred successfully to fresh medium with the same aromatic compound or with toluene as substrate. No growth occurred with o-, m- and p-toluate, benzene, ethylbenzene, styrene and naphthalene. The incubations with the cresols were performed with nitrate instead of manganese oxide as electron acceptor, since the concentration of the cresols decreased rapidly in our controls. This was due to an abiotic reaction of manganese oxide with the cresols. All other tested compounds gave no chemical reaction with amorphous manganese oxide.
3.3 Detection of intermediates
With the presence of the tricarboxylic acid cycle inhibitor fluoroacetate present in a manganese-reducing, toluene-degrading culture of LET-13, 30% of the toluene could be recovered as benzoate and less than 1% as o-cresol.
After the addition of [13C]toluene to culture LET-13, small amounts of 13C-labelled o-cresol (<1% of the added toluene) and 13C-labelled (m+p)-cresol (1–3% of the added toluene) could be detected.
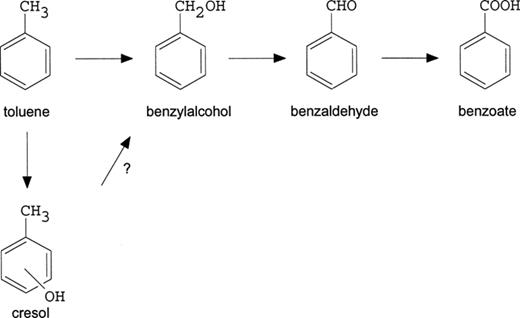
Since a large fraction of the produced cresols reacted abiotically with manganese oxide to undetectable products, we were unable to make a mass balance of the degradation of toluene and the accumulation of intermediates. 30% of the added toluene was transformed to benzoate, and in previous experiments [2] it was shown that approximately 50% of the added toluene was completely mineralized. The initial steps in possible pathways for the toluene degradation in enrichment culture LET-13 are given in Fig. 3.
Initial steps in two proposed pathways for toluene metabolism in LET-13: an oxidation of the methyl group (upper branch) and an oxidation of the aromatic ring (lower branch).
3.4 Dot blot hybridization of the enrichment
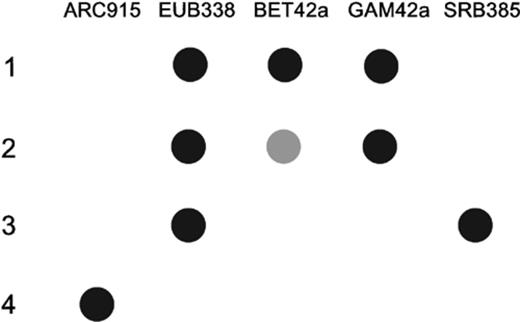
16S rRNA isolated from LET-13 hybridized with a universal eubacterial probe (EUB338) and probes specific for members of the β-subclass (BET42a) and the γ-subclass (GAM42a) of the Proteobacteria (Fig. 4). No hybridization signal was found with the universal archaeal probe (ARC915) and the sulfate-reducing bacterial probe (SRB385). The 16S rRNA of the microorganisms that were used as positive controls gave hybridization signals as expected. Only E. coli also gave a weak signal with BET42a.
Dot blot hybridization of four identical filters containing nucleic acids of four bacterial cultures. 1 μg of 16S rRNA extract from the following bacteria was bound to the filter: 1, LET-13 grown with toluene as substrate and manganese oxide as electron acceptor; 2, Escherichia coli; 3, Synthrophobacter wolinii; 4, Methanosaeta soehngenii. The probes used are described in Table 1.
3.5 DNA molecular analysis (TGGE, cloning, sequence analysis)
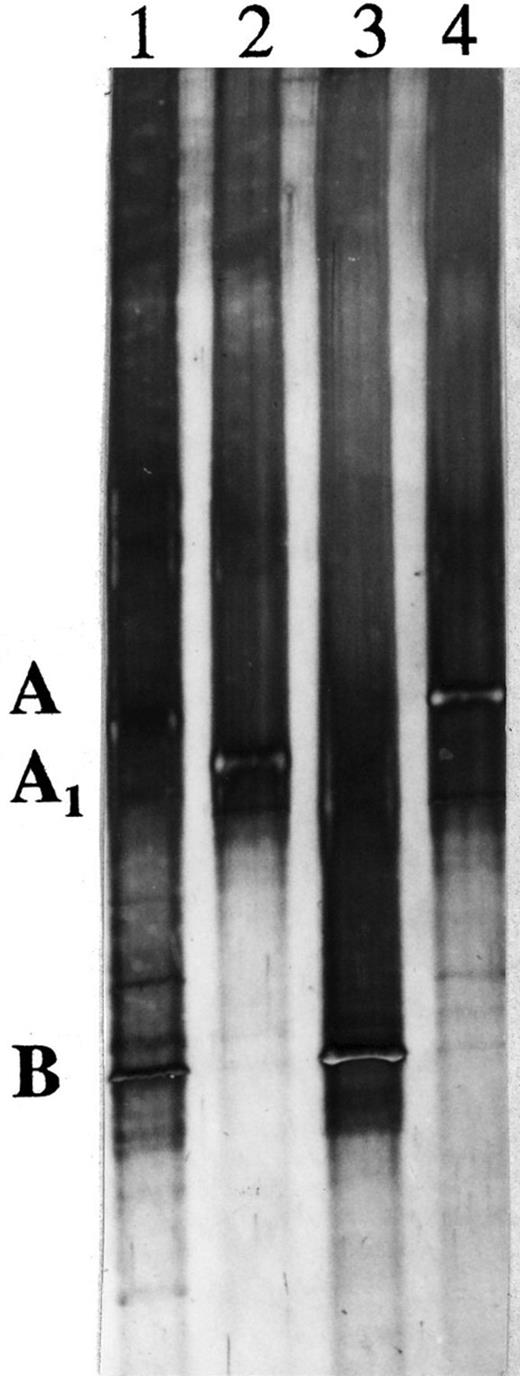
The TGGE profile of the variable regions V6–V8 of LET-13 consisted of two strong bands (A and B) with a large difference in retention time and several weak bands near these two bands (Fig. 5, lane 1). PCR products from the clones I, II and III (Fig. 5, lanes 2, 3 and 4) gave bands with equal positions in the TGGE profile of LET-13 (Fig. 5, bands A, A1 and B). TGGE analyses of the 16S rRNA fragments corresponding to the variable regions V1–V3 and V4–V6 resulted in a TGGE profile with bands at comparable retention times as A, A1 and B (data not shown).
Silver-stained TGGE profile of product originating from V6–V8 region 16S rRNA genes extracted from LET-13: 1, LET-13; 2, clone I; 3, clone II; 4, clone III. The strong bands are indicated as A and B, respectively, and a weak band as A1.
The 16S rRNA gene sequence analysis was restricted to the Eubacterial domain. The Archaeal domain was not analyzed since hybridization of the DNA with the archaebacterial probe did not reveal the presence of Archaea in LET-13. The nucleic acid sequence of 740 bp of clone I was determined, which comprised the variable regions V1–V4 and a long stretch of conserved region. The sequences of this clone was assigned to the Bacteroides/Cytophaga branch group with similarity levels of 70% and 80% with Cytophaga and Bacteroides genera, respectively. The nucleic acid sequence of two stretches of clone II were determined. It comprised the variable regions V3–V6 (500 bp) and V7–V9 (500 bp). The results from the comparison analysis of both stretches indicate members of the β-subclass of the Proteobacteria. The highest similarity (91%) was found with the genus Azoarcus. Two stretches (V1–V4 and V7–V9) of clone III showed similarity values between 80 and 85% with Cytophaga and Bacteroides genera. Moreover, clone I showed a similarity of more than 95% with clone III.
4 Discussion
The newly enriched culture LET-13 is the first anaerobic bacterial enrichment culture that is able to couple the mineralization of toluene to the reduction of manganese oxide. We have demonstrated in earlier experiments [2] that the low degradation rate of toluene was mainly due to the use of the hardly soluble manganese oxide as electron acceptor (MnO2). This rate could only be increased slightly by the addition of organic ligands. Routine transfers were done after 30–60 days of incubation, when approximately 100 μmol of toluene had been degraded. A culture was obtained that consisted of short, motile, rod-shaped bacteria that looked identical. As all attempts to isolate a pure culture failed, we decided to proceed with a further characterization of the enriched culture LET-13, which was stable for more than 2 years.
In addition to manganese (IV), enrichment culture LET-13 was also able to use nitrate and oxygen as electron acceptor. The degradation rate of toluene with these alternative electron acceptors was much higher than when manganese oxide was used (results not shown). The redox potential of these electron acceptors is in the same range as Mn(IV), but nitrate and oxygen are more soluble and might be better available to microorganisms than Mn(IV). Thiosulfate and fumarate could not be used as electron acceptors.
We have routinely tested cultivations of LET-13 on benzoate. Since benzoate was degraded faster than toluene, a faster growth of the bacteria would be the result. However, after cultivating four generations with benzoate, the bacteria had lost their toluene-degrading activity completely.
The ability of LET-13 to degrade specific monoaromatic hydrocarbons, and the detection of large amounts of benzoate and minor amounts of o- and (m+p)-cresol as intermediates in the degradation of toluene, when an inhibitor (fluoroacetate) was added, suggest the presence of at least two pathways. A major part of the toluene was degraded to benzoate, possibly via a methyl oxidation to benzylalcohol and benzaldehyde. We were not able to detect these compounds as intermediates, but both could be used as growth substrates. In a denitrifying, toluene-degrading Pseudomonas strain, both compounds were found as intracellular products [8]. The oxidation of toluene via benzylalcohol and benzaldehyde has also been demonstrated in several other denitrifying strains [16,17,41,42]. Furthermore, benzylalcohol dehydrogenase and benzoyl-CoA reductase have been purified from cell extracts of Thauera aromatica grown on toluene [43,44]. No information is available in the literature about the first enzyme that converts toluene into benzylalcohol, toluene methyl hydroxylase [7,45]. LET-13 is able to grow on o-, m- and p-cresol with nitrate as electron acceptor. The transformation of p-cresol has also been demonstrated before in a methanogenic enrichment culture [46], but could not be detected in a denitrifying or in a sulfate-reducing toluene-degrading bacterium [7,25]. With toluene and manganese oxide, small amounts of o-cresol and (m+p)-cresol were found in the culture LET-13. No quantitative estimates could be made, since the cresols underwent a fast abiotic reaction with manganese oxide. The formation of polymeric oxidation products is the most logical result of the abiotic reaction of the cresols and manganese oxide [47].
The proposed pathways for toluene degradation by LET-13 as given in Fig. 3 have also been postulated by Lovley et al. [20] to occur in G. metallireducens, with iron as electron acceptor.
Two other pathways have also been suggested. Toluene can be mineralized by an oxidative addition of acetyl-CoA to the methyl group of toluene, followed by β-oxidation of the formed phenylpropionyl-CoA [11,21]. The addition of fumarate to the methyl group to form benzylsuccinate and benzoyl-CoA was demonstrated in the denitrifying bacterium T. auromatica[31]. Since benzoate and cresol are not intermediates in these pathways, it is unlikely that these routes are significant pathways in LET-13.
Analyses of rRNA of LET-13 indicated the absence of methanogens and the presence of members of the β- and γ-subclasses of the Proteobacteria. The high homology of the probes for the β- and γ-subclasses of the Proteobacteria makes it difficult to distinguish between these two groups of bacteria on the basis of dot blot hybridizations. It has been shown in other studies that E. coli and other members of the γ-subclass of the Proteobacteria gave a weak hybridization signal with BET42a while members of the β-subclass can hybridize with GAM42a as well [48].
Sequence analyses of cloned rDNA of the enrichment culture resulted in at least three different sequences. Clones I and III are closely related (>95% similarity) and might belong to the same species or even the same bacterium. It is known that sequences of 16S rRNA genes can differ from one copy to another in the same bacterium [49]. In addition, sequence analyses showed that LET-13 contains two major bacterial types. One can be ascribed to the group of Bacteroides-Cytophaga and the other to the β-subclass of the Proteobacteria (e.g., Azoarcus). Both Bacteroides-Cytophaga and Azoarcus can grow anaerobically, are motile rods and may possess flagellae [50,51]. Azoarcus strains used to be characterized as strictly aerobic, but recently, anaerobic denitrifying, toluene-degrading bacteria have been described as members of Azoarcus sp. [19]. Since the level of similarity of the two major groups of bacteria in LET-13 with known sequences for Bacteroides-Cytophaga and Azoarcus is less than 95%, it is likely that both bacteria in LET-13 belong to unknown genera. It has been demonstrated that the similarity for the assignment of bacteria at the genus level is 95%[52]. We could not clarify which type of bacterium is dominant in LET-13, nor which one is involved in the reduction of manganese oxide.
Several manganese-reducing bacteria have been described, but they are not closely related to the ones in LET-13. The manganese-reducing bacteria Shewanella putrefaciens and G. metallireducens were classified as members of the γ-subclass and the δ-subclass of the Proteobacteria, respectively [5,6]. The latter was found to be closely related to Desulfuromonas acetoxidans.
We have demonstrated that LET-13 consists of two major groups of bacteria. The bacteria are related to Bacteroides-Cytophaga types and members of the β-subclass of the Proteobacteria, but it is more likely that they belong to yet unknown genera. Whether each group of these bacteria in LET-13 degrades toluene by one pathway, or possesses the enzymes to use two different pathways is not yet known. Furthermore, it is possible that both groups of bacteria perform the mineralization of toluene in a syntrophic association.
Acknowledgements
We would like to thank Francis Koene-Cottaar for the electron micrographs, Antoon D.L. Akkermans and Wilma M. Akkermans-van Vliet for their help with the molecular analyses, and Tim Grotenhuis of the Department of Environmental Sciene and Technology for providing [13C]toluene. This work was supported by a grant from DSM Research B.V., The Netherlands and by a fellowship to M.B. from Wageningen Agricultural University, The Netherlands.
References
Author notes
Present address: EAWAG, 8600 Dübendorf, Switzerland.