-
PDF
- Split View
-
Views
-
Cite
Cite
GLYNIS V. CRON, KEVIN BALKWILL, ERIC B. KNOX, Biogeography, rarity and endemism in Cineraria (Asteraceae-Senecioneae), Botanical Journal of the Linnean Society, Volume 160, Issue 2, June 2009, Pages 130–148, https://doi.org/10.1111/j.1095-8339.2009.00967.x
Close - Share Icon Share
Abstract
The biogeography of Cineraria (Asteraceae, Senecioneae) is assessed using a chorological approach in terms of its distribution, centres of diversity and endemism. Rare species are identified and categorised according to Rabinowitz's criteria and causes for rarity in the genus are investigated. The conservation status of the species is assessed according to IUCN criteria for Red List categories and compared to levels of rarity. The main phytogeographic affinity of Cineraria is Afromontane in association with seven recognised centres of endemism in South Africa, four in tropical Africa, in Ethiopia and in Madagascar. Fifteen species are endemic and six are near-endemic to a specific centre of endemism or mountain range. Seventy four percent of Cineraria spp. are endemic to southern Africa with the centre of diversity in the KwaZulu-Natal Midlands, South Africa. The rarest species number 11; of these eight are endangered or vulnerable according to IUCN Red Data Criteria and three are data deficient. Causes of rarity in Cineraria are related to narrow habitat specificity, notably soil or rock type and/or altitudinal range. Paired comparisons of the 11 rarest and commonest species reveal no convincing causal links to morphological, reproductive or life history strategy attributes in Cineraria.
INTRODUCTION
Cineraria L. of Senecioneae Cass. (Asteraceae) comprises 35 species and is essentially an African genus, with one species [C. anampoza (Baker) Baker] endemic to Madagascar and another (C. abyssinica Sch. Bip. ex A.Rich) extending from Ethiopia into the mountains of Yemen and Saudi Arabia (Table 1). Cineraria is predominantly Afromontane, but descends to sea level in the Western Cape [C. geifolia (L.) L. and C. angulosa Lam.], thereby exhibiting an ‘African track’ distribution pattern (Linder, Meadows & Cowling, 1992). Cineraria shows a distribution pattern similar to several other genera in Asteraceae, namely, Athrixia Ker Gawl., Felicia Cass., Osteospermum L., Pentzia Thunb., Stoebe L. and Ursinia Gaertn. (Koekemoer, 1996).
Ecology, distribution, rarity and IUCN assessment of the species of Cineraria
| Species of Cineraria with authorities . | N 1DS† . | N QDS‡ . | Distribution§ . | Altitude range (m) . | Soil types¶ . | Centres of endemism/floristic region†† . | Rarity‡‡ . | Global IUCN assessment§§ . |
|---|---|---|---|---|---|---|---|---|
| C. abyssinica Sch. Bip. ex A. Rich. | 15 | 23 | Ethiopia, Eritrea, Yemen, Saudi Arabia | 2300–4100 | LS + loam | Bale Mts, Simen Mts | N | DD |
| C. albicans N.E.Br. | 9 | 16 | KZN, EC | 170–2600 | NGS + MFS | Albany, DA | N | LC |
| C. alchemilloides DC. | 14 | 19 | NC, WC, Namibia | 800–1750 | Q, G | CFR, Brandberg | * | LC |
| C. anampoza (Baker) Baker | 6 | 10 | Madagascar | 1200–2600 | Q, clay, laterites | (Madagascar) | N? | DD |
| C. angulosa L'Hér. | 2 | 2 | WC | c. 30 | G | CFR | *** | EN B1ab (ii, iii, iv) |
| C. aspera Thunb. | 49 | 99 | SA, Lesotho | 1400–2600 | Q, D | Albany, DA | N | LC |
| C. atriplicifolia DC. | 3 | 7 | KZN | 30–1200 | NGS | KZN Midlands in M-P | ** | VU B1ab (iii) |
| C. austrotransvaalensis Cron | 5 | 9 | Gauteng, NW, MP | 1400–1700 | Q, D | * | NT B1ab (iii) | |
| C. canescens Wendl. ex Link | 7 | 12 | NC, WC, Namibia | 570–1600 (–2200) | G | Kamiesberg in CFR | ** | LC |
| C. cyanomontana Cron | 1 | 1 | Blouberg (L) | 1700–2000 | Q | SC (Blouberg) | *** | EN D |
| C. decipiens Harv. | 9 | 11 | KZN, Swaziland | 100–1150 | MFS, G, NGS? | M-P | * | LC |
| C. deltoidea Sond. | 62 | 220 | Eth, EA, Malawi, L, MP? KZN, Zambia, Zimbabwe | 500–4300 | NGS, Dol, B | Bale Mts, Imatong, Mt Kenya, Mt Mlanje, Ch, SC, KZN Midlands in M-P | N | LC |
| C. dryogeton Cron | 1 | 1 | KZN | 300–400 | MFS | Pondoland (M-P) | *** | VU D2 |
| C. erodioides DC. | 38 | 101 | SA, Lesotho | 100–3300 | B, NGS, Q? | DA, Albany, SC, W | N | LC |
| C. erosa (Thunb.) Willd. | 9 | 23 | WC, NC | 300–1750 | G, CSS | CFR | * | LC |
| C. foliosa O.Hoffm. | 1 | 1 | Kitulo, Tanzania | 2700 | SH Tanzania | *** | DD | |
| C. geifolia (L.) L. | 6 | 28 | WC | 10–500 | Coastal sands | CFR | * | LC |
| C. geraniifolia DC. | 21 | 46 | EC, KZN, MP | 1300–2550 | B, Dol, NGS? | DA, Albany | * | LC |
| C. glandulosa Cron | 3 | 5 | KZN | 630–1800 | NGS | KZN Midlands in M-P | *** | VU D2 |
| C. grandibracteata Hilliard | 4 | 9 | KZN, EC | 450–1900 | Dol | KZN Midlands in M-P | ** | LC |
| C. huilensis Cron | 2 | 5 | Angola | 1700–2400 | Q/G? | Huila | *** | DD |
| C. lobata L'Hér. | 29 | 64 | WC, EC, NC, L | 10–1800 | CSS, Q, G | CFR, Albany, SC | N | LC |
| C. longipes S.Moore | 2 | 5 | Gauteng | 1500–1850 | B | *** | VU D2 | |
| C. lyratiformis Cron | 35 | 88 | SA, Lesotho | 1250–2450 | Q, Dol | N M in M-P, Albany, DA | N | LC |
| C. magnicephala Cron | 2 | 2 | Malawi | 1900–2030 | Q? | *** | DD | |
| C. mazoensis S.Moore | 12 | 14 | Zimbabwe, Zambia, M | 1100–1905 | Q, G | * | DD | |
| C. mollis E.Mey. ex DC. | 19 | 33 | WC, EC, NC, Lesotho | 1600–2550 | B, Dol, NGS | DA | N | LC |
| C. ngwenyensis Cron | 1 | 1 | Swaziland | 1500–1700 | Q | Barberton | *** | VU D2 |
| C. parvifolia Burtt Davy | 11 | 17 | Gauteng, NW, L, MP | 1250–1600 (–2000) | Q, sandy loams | N | LC | |
| C. pinnata O.Hoffm. | 5 | 8 | KZN, Moz | 20–70 | Coastal sands | Maputaland in M-P | * | LC (Globally), NT D2 (SA) |
| C. platycarpa DC. | 14 | 31 | WC, EC | 20–900 | Bokkeveld & Ecca shales | Little Karoo (in CFR) | * | LC |
| C. pulchra Cron | 4 | 7 | Zimbabwe, Moz | 1700–2540 | Q, G | Chimanimani-Nyanga | * | LC |
| C. saxifraga DC. | 6 | 18 | EC, (FS) | 400–1000 | Q | Albany | * | LC |
| C. vagans Hilliard | 1 | 2 | EC | 1380–1750 | D | Albany | *** | EN B1ab(iii) |
| C. vallis-pacis Dinter ex Merxm. | 10 | 23 | NC, Namibia | 1140–2000 | D (TSG), Kalahari sands | GWC | N | LC |
| Species of Cineraria with authorities . | N 1DS† . | N QDS‡ . | Distribution§ . | Altitude range (m) . | Soil types¶ . | Centres of endemism/floristic region†† . | Rarity‡‡ . | Global IUCN assessment§§ . |
|---|---|---|---|---|---|---|---|---|
| C. abyssinica Sch. Bip. ex A. Rich. | 15 | 23 | Ethiopia, Eritrea, Yemen, Saudi Arabia | 2300–4100 | LS + loam | Bale Mts, Simen Mts | N | DD |
| C. albicans N.E.Br. | 9 | 16 | KZN, EC | 170–2600 | NGS + MFS | Albany, DA | N | LC |
| C. alchemilloides DC. | 14 | 19 | NC, WC, Namibia | 800–1750 | Q, G | CFR, Brandberg | * | LC |
| C. anampoza (Baker) Baker | 6 | 10 | Madagascar | 1200–2600 | Q, clay, laterites | (Madagascar) | N? | DD |
| C. angulosa L'Hér. | 2 | 2 | WC | c. 30 | G | CFR | *** | EN B1ab (ii, iii, iv) |
| C. aspera Thunb. | 49 | 99 | SA, Lesotho | 1400–2600 | Q, D | Albany, DA | N | LC |
| C. atriplicifolia DC. | 3 | 7 | KZN | 30–1200 | NGS | KZN Midlands in M-P | ** | VU B1ab (iii) |
| C. austrotransvaalensis Cron | 5 | 9 | Gauteng, NW, MP | 1400–1700 | Q, D | * | NT B1ab (iii) | |
| C. canescens Wendl. ex Link | 7 | 12 | NC, WC, Namibia | 570–1600 (–2200) | G | Kamiesberg in CFR | ** | LC |
| C. cyanomontana Cron | 1 | 1 | Blouberg (L) | 1700–2000 | Q | SC (Blouberg) | *** | EN D |
| C. decipiens Harv. | 9 | 11 | KZN, Swaziland | 100–1150 | MFS, G, NGS? | M-P | * | LC |
| C. deltoidea Sond. | 62 | 220 | Eth, EA, Malawi, L, MP? KZN, Zambia, Zimbabwe | 500–4300 | NGS, Dol, B | Bale Mts, Imatong, Mt Kenya, Mt Mlanje, Ch, SC, KZN Midlands in M-P | N | LC |
| C. dryogeton Cron | 1 | 1 | KZN | 300–400 | MFS | Pondoland (M-P) | *** | VU D2 |
| C. erodioides DC. | 38 | 101 | SA, Lesotho | 100–3300 | B, NGS, Q? | DA, Albany, SC, W | N | LC |
| C. erosa (Thunb.) Willd. | 9 | 23 | WC, NC | 300–1750 | G, CSS | CFR | * | LC |
| C. foliosa O.Hoffm. | 1 | 1 | Kitulo, Tanzania | 2700 | SH Tanzania | *** | DD | |
| C. geifolia (L.) L. | 6 | 28 | WC | 10–500 | Coastal sands | CFR | * | LC |
| C. geraniifolia DC. | 21 | 46 | EC, KZN, MP | 1300–2550 | B, Dol, NGS? | DA, Albany | * | LC |
| C. glandulosa Cron | 3 | 5 | KZN | 630–1800 | NGS | KZN Midlands in M-P | *** | VU D2 |
| C. grandibracteata Hilliard | 4 | 9 | KZN, EC | 450–1900 | Dol | KZN Midlands in M-P | ** | LC |
| C. huilensis Cron | 2 | 5 | Angola | 1700–2400 | Q/G? | Huila | *** | DD |
| C. lobata L'Hér. | 29 | 64 | WC, EC, NC, L | 10–1800 | CSS, Q, G | CFR, Albany, SC | N | LC |
| C. longipes S.Moore | 2 | 5 | Gauteng | 1500–1850 | B | *** | VU D2 | |
| C. lyratiformis Cron | 35 | 88 | SA, Lesotho | 1250–2450 | Q, Dol | N M in M-P, Albany, DA | N | LC |
| C. magnicephala Cron | 2 | 2 | Malawi | 1900–2030 | Q? | *** | DD | |
| C. mazoensis S.Moore | 12 | 14 | Zimbabwe, Zambia, M | 1100–1905 | Q, G | * | DD | |
| C. mollis E.Mey. ex DC. | 19 | 33 | WC, EC, NC, Lesotho | 1600–2550 | B, Dol, NGS | DA | N | LC |
| C. ngwenyensis Cron | 1 | 1 | Swaziland | 1500–1700 | Q | Barberton | *** | VU D2 |
| C. parvifolia Burtt Davy | 11 | 17 | Gauteng, NW, L, MP | 1250–1600 (–2000) | Q, sandy loams | N | LC | |
| C. pinnata O.Hoffm. | 5 | 8 | KZN, Moz | 20–70 | Coastal sands | Maputaland in M-P | * | LC (Globally), NT D2 (SA) |
| C. platycarpa DC. | 14 | 31 | WC, EC | 20–900 | Bokkeveld & Ecca shales | Little Karoo (in CFR) | * | LC |
| C. pulchra Cron | 4 | 7 | Zimbabwe, Moz | 1700–2540 | Q, G | Chimanimani-Nyanga | * | LC |
| C. saxifraga DC. | 6 | 18 | EC, (FS) | 400–1000 | Q | Albany | * | LC |
| C. vagans Hilliard | 1 | 2 | EC | 1380–1750 | D | Albany | *** | EN B1ab(iii) |
| C. vallis-pacis Dinter ex Merxm. | 10 | 23 | NC, Namibia | 1140–2000 | D (TSG), Kalahari sands | GWC | N | LC |
1DS: one degree square.
QDS: quarter degree square.
Distribution: EC, Eastern Cape; Eth, Ethiopia; FS, Free State; KZN, KwaZulu-Natal; L, Limpopo Province; M, Malawi; Moz, Mozambique; MP, Mpumalanga; NC, Northern Cape; SA, South Africa (widespread); WC, Western Cape.
Soil types/geological formations: B, basalt; CSS, Cape supergroup sandstone; D, dolomite; Dol, dolerite; G, granite; LS, limestone; MFS, Msikaba Formation sandstone; NGS, Natal group sandstone; Q, quartzite; TSG, Transvaal Supergroup.
Centres of Endemism/Floristic Regions: CFR, Cape Floristic Region; DA, Drakensberg Alpine Centre; KZN, KwaZulu-Natal; GWC, Griqualand West Centre; M-P, Maputaland–Pondoland Region; SC, Soutpansberg Centre; W, Wolkberg Centre.
Rarity: N, common, not rare;
intermediate;
fairly rare;
rare.
IUCN Red Data Categories: DD, data deficient; EN, endangered; LC, least concern; VU, vulnerable; NT, near threatened.
Ecology, distribution, rarity and IUCN assessment of the species of Cineraria
| Species of Cineraria with authorities . | N 1DS† . | N QDS‡ . | Distribution§ . | Altitude range (m) . | Soil types¶ . | Centres of endemism/floristic region†† . | Rarity‡‡ . | Global IUCN assessment§§ . |
|---|---|---|---|---|---|---|---|---|
| C. abyssinica Sch. Bip. ex A. Rich. | 15 | 23 | Ethiopia, Eritrea, Yemen, Saudi Arabia | 2300–4100 | LS + loam | Bale Mts, Simen Mts | N | DD |
| C. albicans N.E.Br. | 9 | 16 | KZN, EC | 170–2600 | NGS + MFS | Albany, DA | N | LC |
| C. alchemilloides DC. | 14 | 19 | NC, WC, Namibia | 800–1750 | Q, G | CFR, Brandberg | * | LC |
| C. anampoza (Baker) Baker | 6 | 10 | Madagascar | 1200–2600 | Q, clay, laterites | (Madagascar) | N? | DD |
| C. angulosa L'Hér. | 2 | 2 | WC | c. 30 | G | CFR | *** | EN B1ab (ii, iii, iv) |
| C. aspera Thunb. | 49 | 99 | SA, Lesotho | 1400–2600 | Q, D | Albany, DA | N | LC |
| C. atriplicifolia DC. | 3 | 7 | KZN | 30–1200 | NGS | KZN Midlands in M-P | ** | VU B1ab (iii) |
| C. austrotransvaalensis Cron | 5 | 9 | Gauteng, NW, MP | 1400–1700 | Q, D | * | NT B1ab (iii) | |
| C. canescens Wendl. ex Link | 7 | 12 | NC, WC, Namibia | 570–1600 (–2200) | G | Kamiesberg in CFR | ** | LC |
| C. cyanomontana Cron | 1 | 1 | Blouberg (L) | 1700–2000 | Q | SC (Blouberg) | *** | EN D |
| C. decipiens Harv. | 9 | 11 | KZN, Swaziland | 100–1150 | MFS, G, NGS? | M-P | * | LC |
| C. deltoidea Sond. | 62 | 220 | Eth, EA, Malawi, L, MP? KZN, Zambia, Zimbabwe | 500–4300 | NGS, Dol, B | Bale Mts, Imatong, Mt Kenya, Mt Mlanje, Ch, SC, KZN Midlands in M-P | N | LC |
| C. dryogeton Cron | 1 | 1 | KZN | 300–400 | MFS | Pondoland (M-P) | *** | VU D2 |
| C. erodioides DC. | 38 | 101 | SA, Lesotho | 100–3300 | B, NGS, Q? | DA, Albany, SC, W | N | LC |
| C. erosa (Thunb.) Willd. | 9 | 23 | WC, NC | 300–1750 | G, CSS | CFR | * | LC |
| C. foliosa O.Hoffm. | 1 | 1 | Kitulo, Tanzania | 2700 | SH Tanzania | *** | DD | |
| C. geifolia (L.) L. | 6 | 28 | WC | 10–500 | Coastal sands | CFR | * | LC |
| C. geraniifolia DC. | 21 | 46 | EC, KZN, MP | 1300–2550 | B, Dol, NGS? | DA, Albany | * | LC |
| C. glandulosa Cron | 3 | 5 | KZN | 630–1800 | NGS | KZN Midlands in M-P | *** | VU D2 |
| C. grandibracteata Hilliard | 4 | 9 | KZN, EC | 450–1900 | Dol | KZN Midlands in M-P | ** | LC |
| C. huilensis Cron | 2 | 5 | Angola | 1700–2400 | Q/G? | Huila | *** | DD |
| C. lobata L'Hér. | 29 | 64 | WC, EC, NC, L | 10–1800 | CSS, Q, G | CFR, Albany, SC | N | LC |
| C. longipes S.Moore | 2 | 5 | Gauteng | 1500–1850 | B | *** | VU D2 | |
| C. lyratiformis Cron | 35 | 88 | SA, Lesotho | 1250–2450 | Q, Dol | N M in M-P, Albany, DA | N | LC |
| C. magnicephala Cron | 2 | 2 | Malawi | 1900–2030 | Q? | *** | DD | |
| C. mazoensis S.Moore | 12 | 14 | Zimbabwe, Zambia, M | 1100–1905 | Q, G | * | DD | |
| C. mollis E.Mey. ex DC. | 19 | 33 | WC, EC, NC, Lesotho | 1600–2550 | B, Dol, NGS | DA | N | LC |
| C. ngwenyensis Cron | 1 | 1 | Swaziland | 1500–1700 | Q | Barberton | *** | VU D2 |
| C. parvifolia Burtt Davy | 11 | 17 | Gauteng, NW, L, MP | 1250–1600 (–2000) | Q, sandy loams | N | LC | |
| C. pinnata O.Hoffm. | 5 | 8 | KZN, Moz | 20–70 | Coastal sands | Maputaland in M-P | * | LC (Globally), NT D2 (SA) |
| C. platycarpa DC. | 14 | 31 | WC, EC | 20–900 | Bokkeveld & Ecca shales | Little Karoo (in CFR) | * | LC |
| C. pulchra Cron | 4 | 7 | Zimbabwe, Moz | 1700–2540 | Q, G | Chimanimani-Nyanga | * | LC |
| C. saxifraga DC. | 6 | 18 | EC, (FS) | 400–1000 | Q | Albany | * | LC |
| C. vagans Hilliard | 1 | 2 | EC | 1380–1750 | D | Albany | *** | EN B1ab(iii) |
| C. vallis-pacis Dinter ex Merxm. | 10 | 23 | NC, Namibia | 1140–2000 | D (TSG), Kalahari sands | GWC | N | LC |
| Species of Cineraria with authorities . | N 1DS† . | N QDS‡ . | Distribution§ . | Altitude range (m) . | Soil types¶ . | Centres of endemism/floristic region†† . | Rarity‡‡ . | Global IUCN assessment§§ . |
|---|---|---|---|---|---|---|---|---|
| C. abyssinica Sch. Bip. ex A. Rich. | 15 | 23 | Ethiopia, Eritrea, Yemen, Saudi Arabia | 2300–4100 | LS + loam | Bale Mts, Simen Mts | N | DD |
| C. albicans N.E.Br. | 9 | 16 | KZN, EC | 170–2600 | NGS + MFS | Albany, DA | N | LC |
| C. alchemilloides DC. | 14 | 19 | NC, WC, Namibia | 800–1750 | Q, G | CFR, Brandberg | * | LC |
| C. anampoza (Baker) Baker | 6 | 10 | Madagascar | 1200–2600 | Q, clay, laterites | (Madagascar) | N? | DD |
| C. angulosa L'Hér. | 2 | 2 | WC | c. 30 | G | CFR | *** | EN B1ab (ii, iii, iv) |
| C. aspera Thunb. | 49 | 99 | SA, Lesotho | 1400–2600 | Q, D | Albany, DA | N | LC |
| C. atriplicifolia DC. | 3 | 7 | KZN | 30–1200 | NGS | KZN Midlands in M-P | ** | VU B1ab (iii) |
| C. austrotransvaalensis Cron | 5 | 9 | Gauteng, NW, MP | 1400–1700 | Q, D | * | NT B1ab (iii) | |
| C. canescens Wendl. ex Link | 7 | 12 | NC, WC, Namibia | 570–1600 (–2200) | G | Kamiesberg in CFR | ** | LC |
| C. cyanomontana Cron | 1 | 1 | Blouberg (L) | 1700–2000 | Q | SC (Blouberg) | *** | EN D |
| C. decipiens Harv. | 9 | 11 | KZN, Swaziland | 100–1150 | MFS, G, NGS? | M-P | * | LC |
| C. deltoidea Sond. | 62 | 220 | Eth, EA, Malawi, L, MP? KZN, Zambia, Zimbabwe | 500–4300 | NGS, Dol, B | Bale Mts, Imatong, Mt Kenya, Mt Mlanje, Ch, SC, KZN Midlands in M-P | N | LC |
| C. dryogeton Cron | 1 | 1 | KZN | 300–400 | MFS | Pondoland (M-P) | *** | VU D2 |
| C. erodioides DC. | 38 | 101 | SA, Lesotho | 100–3300 | B, NGS, Q? | DA, Albany, SC, W | N | LC |
| C. erosa (Thunb.) Willd. | 9 | 23 | WC, NC | 300–1750 | G, CSS | CFR | * | LC |
| C. foliosa O.Hoffm. | 1 | 1 | Kitulo, Tanzania | 2700 | SH Tanzania | *** | DD | |
| C. geifolia (L.) L. | 6 | 28 | WC | 10–500 | Coastal sands | CFR | * | LC |
| C. geraniifolia DC. | 21 | 46 | EC, KZN, MP | 1300–2550 | B, Dol, NGS? | DA, Albany | * | LC |
| C. glandulosa Cron | 3 | 5 | KZN | 630–1800 | NGS | KZN Midlands in M-P | *** | VU D2 |
| C. grandibracteata Hilliard | 4 | 9 | KZN, EC | 450–1900 | Dol | KZN Midlands in M-P | ** | LC |
| C. huilensis Cron | 2 | 5 | Angola | 1700–2400 | Q/G? | Huila | *** | DD |
| C. lobata L'Hér. | 29 | 64 | WC, EC, NC, L | 10–1800 | CSS, Q, G | CFR, Albany, SC | N | LC |
| C. longipes S.Moore | 2 | 5 | Gauteng | 1500–1850 | B | *** | VU D2 | |
| C. lyratiformis Cron | 35 | 88 | SA, Lesotho | 1250–2450 | Q, Dol | N M in M-P, Albany, DA | N | LC |
| C. magnicephala Cron | 2 | 2 | Malawi | 1900–2030 | Q? | *** | DD | |
| C. mazoensis S.Moore | 12 | 14 | Zimbabwe, Zambia, M | 1100–1905 | Q, G | * | DD | |
| C. mollis E.Mey. ex DC. | 19 | 33 | WC, EC, NC, Lesotho | 1600–2550 | B, Dol, NGS | DA | N | LC |
| C. ngwenyensis Cron | 1 | 1 | Swaziland | 1500–1700 | Q | Barberton | *** | VU D2 |
| C. parvifolia Burtt Davy | 11 | 17 | Gauteng, NW, L, MP | 1250–1600 (–2000) | Q, sandy loams | N | LC | |
| C. pinnata O.Hoffm. | 5 | 8 | KZN, Moz | 20–70 | Coastal sands | Maputaland in M-P | * | LC (Globally), NT D2 (SA) |
| C. platycarpa DC. | 14 | 31 | WC, EC | 20–900 | Bokkeveld & Ecca shales | Little Karoo (in CFR) | * | LC |
| C. pulchra Cron | 4 | 7 | Zimbabwe, Moz | 1700–2540 | Q, G | Chimanimani-Nyanga | * | LC |
| C. saxifraga DC. | 6 | 18 | EC, (FS) | 400–1000 | Q | Albany | * | LC |
| C. vagans Hilliard | 1 | 2 | EC | 1380–1750 | D | Albany | *** | EN B1ab(iii) |
| C. vallis-pacis Dinter ex Merxm. | 10 | 23 | NC, Namibia | 1140–2000 | D (TSG), Kalahari sands | GWC | N | LC |
1DS: one degree square.
QDS: quarter degree square.
Distribution: EC, Eastern Cape; Eth, Ethiopia; FS, Free State; KZN, KwaZulu-Natal; L, Limpopo Province; M, Malawi; Moz, Mozambique; MP, Mpumalanga; NC, Northern Cape; SA, South Africa (widespread); WC, Western Cape.
Soil types/geological formations: B, basalt; CSS, Cape supergroup sandstone; D, dolomite; Dol, dolerite; G, granite; LS, limestone; MFS, Msikaba Formation sandstone; NGS, Natal group sandstone; Q, quartzite; TSG, Transvaal Supergroup.
Centres of Endemism/Floristic Regions: CFR, Cape Floristic Region; DA, Drakensberg Alpine Centre; KZN, KwaZulu-Natal; GWC, Griqualand West Centre; M-P, Maputaland–Pondoland Region; SC, Soutpansberg Centre; W, Wolkberg Centre.
Rarity: N, common, not rare;
intermediate;
fairly rare;
rare.
IUCN Red Data Categories: DD, data deficient; EN, endangered; LC, least concern; VU, vulnerable; NT, near threatened.
Most species of Cineraria occur in southern Africa, with only a few extending into the equatorial zone and/or northern hemisphere. Most have a restricted distribution and many are associated with recognized areas of endemism. The most widespread species is C. deltoidea Sond. (including plants previously known as C. grandiflora Vatke and 12 other synonyms), which occurs throughout the mountains of the Rift Valley of Africa, including the high plateau regions of Malawi (Nyika, Zomba, Mulanje and mountains near Blantyre), the eastern highlands of Zimbabwe, the Soutpansberg, KwaZulu-Natal Midlands and forest margins in the Eastern Cape in South Africa (Cron, Balkwill & Knox, 2006a, 2007a). Widespread species in South Africa include C. aspera Thunb., C. erodioides DC. and C. lyratiformis Cron, all of which also occur at high altitude in Lesotho. Cineraria aspera and C. lyratiformis are somewhat weedy species, often growing in disturbed areas. Cineraria abyssinica has also been noted to grow along the edge of fields and near roadsides in Ethiopia and C. anampoza (from Madagascar) has been described as a ‘weedy perennial’. Most other species of Cineraria have fairly specific habitat requirements.
Fifteen species in Cineraria are endemic to specific phytogeographic regions and/or mountains, some are rare and 15 have been categorized (globally) as endangered, vulnerable, near threatened or data deficient according to the IUCN (2001) criteria (Table 1; Scott-Shaw, 1999; Pfab & Victor, 2002; Cron et al., 2006a; Raimondo et al., 2009). For conservation purposes, it is useful to identify rare and endemic species in Cineraria, possible causes for their rarity and associated levels of threat.
Endemism, rarity and the Red Data status of species all provide information that may be useful in prioritizing areas for conservation (Rebelo & Tansley, 1993). Naturally rare species are an important component of endemicity and species diversity (Kruckeberg & Rabinowitz, 1985), whereas threatened rare species are indicative of the most stressed habitats that urgently require protection (Rebelo & Tansley, 1993). Identification of threatened rare species may thus highlight the need for conserving areas of relatively low species richness that might otherwise escape attention if only areas of high species richness are the focus during the conservation prioritization process (Rebelo & Tansley, 1993).
Criteria other than species richness and rarity that are useful in assigning conservation value to taxa and prioritizing areas for conservation are taxic diversity (Vane-Wright, Humphries & Williams, 1991) and phylogenetic diversity (Faith, 1992) based on phylogenetic relationships between taxa. Spatial information on genetic diversity may be incorporated into conservation planning and regional biodiversity assessments by making use of comparative phylogeography to identify evolutionarily distinct communities or areas that maximize phylogenetic diversity (Moritz & Faith, 1998). Phylogenetic diversity has been shown to be decoupled from taxon richness, especially when the phylogenetic base is unbalanced and there is strong phylogeographic structure, such as is present in the biodiversity hotspots of the Western Cape where multiple radiations have occurred (Forest et al., 2007).
Comparisons of traits (‘response variables’) of common and rare species have often been used to investigate the causes and/or consequences of rarity (e.g. Gaston & Kunin, 1997; Orians, 1997; Bevill & Louda, 1999; Farnsworth, 2007). These traits include characters that increase reproductive success (pollination and dispersal, e.g. Rabinowitz & Rapp, 1981; Orians, 1997) and/or survival and longevity and therefore persistence in a habitat, such as life history strategy, size and growth form (e.g. Gaston & Kunin, 1997; Farnsworth, 2007). In addition, specialist features that influence adaptive ability (e.g. various types of trichomes influencing rates of evapotranspiration and/or herbivory) could promote the survival of individuals in specific habitats, at low or high densities, by providing competitive advantage over members of the same or other species. Ecological characteristics of rare and common related plant species could also be the cause of rarity in a species or a consequence of it, i.e. an adaptation to deal with the state of rarity (Kunin & Gaston, 1993). (See Bevill & Louda, 1999 for review.)
The aims of this study are to (1) map the distribution of Cineraria in Africa, (2) identify centres of diversity for Cineraria and analyse its distribution in relation to currently defined phytogeographic units and centres of endemism, (3) identify and compare rare and endemic species, (4) identify possible causes of rarity in the genus and (5) highlight areas with rare threatened species in need of conservation prioritization.
MATERIAL AND METHODS
Distributional data for the 35 species of Cineraria (as listed in Cron et al., 2006a) were obtained from specimens in the following herbaria: BM, BOL, BR, COI, E, EA, G-DC, GRA, J, K, LISC, MO, NBG, NH, NU, P, PRE, PRU, S, SAM, SRGH, TCD, UPS, US, WAG and Z. Information concerning ecology and habitat was compiled from personal observations in the field and from notes on herbarium specimen labels (summarized in Table 1).
A chorological approach was applied at continental and local scales for Cineraria: the number of species in each country (Fig. 1) and the number of species per degree square for southern Africa (Fig. 2) were counted, and the distribution of the six species occurring in the regions of Africa north of 16°S was plotted (Fig. 3). A more detailed analysis was undertaken for southern Africa: the number of one-degree squares and quarter-degree squares (1/16th degree as per Leistner & Morris, 1976) occupied by each species was counted using maps based on the distribution data gathered from specimens (see Cron et al., 2006a for distribution maps for each species) and produced using Mappit Version 2 (Arnold & Oliver, 1996). Distributions were compared with Centres of Endemism as recognized by Van Wyk & Smith (2001) and Beentje, Adams & Davis (1994) and summarized in Tables 1 and 2. A species was considered to be endemic to a region if it occurred only in that region or specific Centre of Endemism and near-endemic if the species occurred (with limited distribution) in an adjacent region outside of the area of reference.
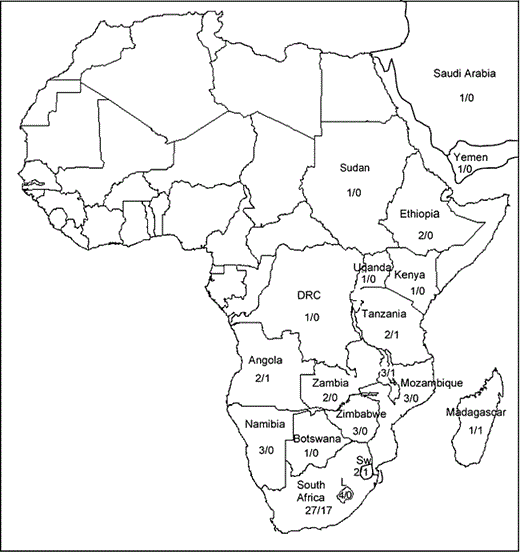
Distribution of Cineraria in Africa and south-west Asia, indicating numbers of species/number of endemics in each country. DRC, Democratic Republic of Congo; L, Lesotho; Sw, Swaziland.
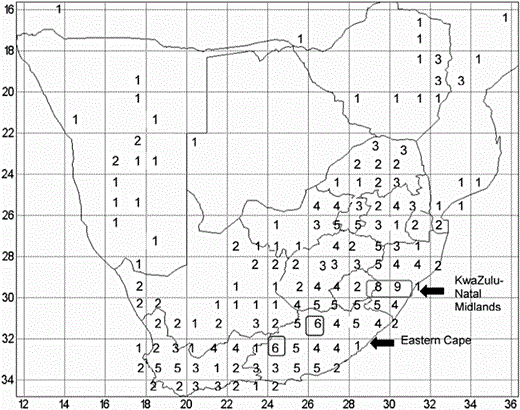
Number of species of Cineraria occurring in each one-degree square in southern Africa. Regions of highest diversity are indicated.
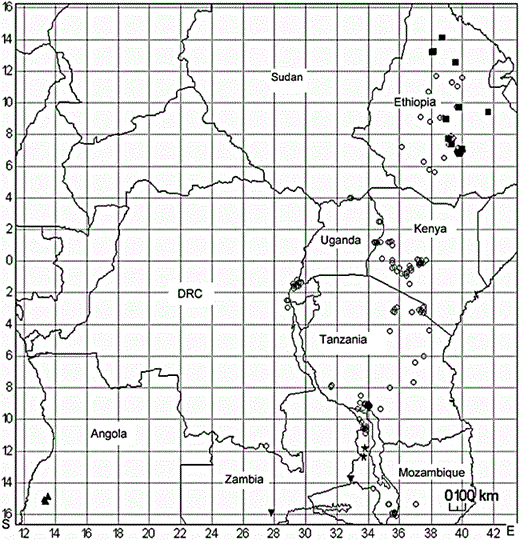
Distribution of the six species of Cineraria occurring north of 16°S: ○ C. deltoidea, ■ C. abyssinica, ◊ C. foliosa, ★ C. magnicephala, ▴ C. huilensis, ▾ C. mazoensis.
Centres of endemism and associated endemic (E) and near-endemic (NE) species and subspecies/varieties of Cineraria
| Centre of endemism . | Species . |
|---|---|
| Angola: Huila: Serra da Chela and surrounds | C. huilensis* (E) |
| Ethiopia: Bale Mts, Simén Mts | C. abyssinica (NE) |
| Madagascar | C. anampoza (E) |
| Malawi: Mzimba District, Lwanjati Hill | C. magnicephala* (E) |
| South Africa: | |
| Albany Centre | C. saxifraga (E), C. vagans* (E); C. lobata subsp. platyptera (E) |
| Barberton Centre | C. ngwenyensis* (E) |
| Cape Floristic Region | C. angulosa* (E), C. erosa (E), C. geifolia (E), C. mollis (NE), C. platycarpa (NE), C. lobata subsp. lasiocaulis (NE), C. lobata subsp. lobata (NE), C. alchemilloides subsp. alchemilloides (NE) |
| Kamiesberg Centre | C. canescens (NE) |
| Maputaland | C. pinnata (E) |
| Pondoland | C. dryogeton* (E), C. albicans (NE), C. decipiens (NE) |
| Soutpansberg Blouberg | C. erodioides var. tomentosa (E), C. lobata subsp. soutpansbergensis (E), C. cyanomontana* (E) |
| Tanzania: Southern Highlands: Kitulo Plateau | C. foliosa* (E) |
| Zimbabwe: Chimanimani–Nyanga | C. pulchra (E) |
| Centre of endemism . | Species . |
|---|---|
| Angola: Huila: Serra da Chela and surrounds | C. huilensis* (E) |
| Ethiopia: Bale Mts, Simén Mts | C. abyssinica (NE) |
| Madagascar | C. anampoza (E) |
| Malawi: Mzimba District, Lwanjati Hill | C. magnicephala* (E) |
| South Africa: | |
| Albany Centre | C. saxifraga (E), C. vagans* (E); C. lobata subsp. platyptera (E) |
| Barberton Centre | C. ngwenyensis* (E) |
| Cape Floristic Region | C. angulosa* (E), C. erosa (E), C. geifolia (E), C. mollis (NE), C. platycarpa (NE), C. lobata subsp. lasiocaulis (NE), C. lobata subsp. lobata (NE), C. alchemilloides subsp. alchemilloides (NE) |
| Kamiesberg Centre | C. canescens (NE) |
| Maputaland | C. pinnata (E) |
| Pondoland | C. dryogeton* (E), C. albicans (NE), C. decipiens (NE) |
| Soutpansberg Blouberg | C. erodioides var. tomentosa (E), C. lobata subsp. soutpansbergensis (E), C. cyanomontana* (E) |
| Tanzania: Southern Highlands: Kitulo Plateau | C. foliosa* (E) |
| Zimbabwe: Chimanimani–Nyanga | C. pulchra (E) |
Indicates ‘Rarest’ species according to Rabinowitz's criteria of narrow distribution range and habitat specificity and small local populations.
Centres of endemism and associated endemic (E) and near-endemic (NE) species and subspecies/varieties of Cineraria
| Centre of endemism . | Species . |
|---|---|
| Angola: Huila: Serra da Chela and surrounds | C. huilensis* (E) |
| Ethiopia: Bale Mts, Simén Mts | C. abyssinica (NE) |
| Madagascar | C. anampoza (E) |
| Malawi: Mzimba District, Lwanjati Hill | C. magnicephala* (E) |
| South Africa: | |
| Albany Centre | C. saxifraga (E), C. vagans* (E); C. lobata subsp. platyptera (E) |
| Barberton Centre | C. ngwenyensis* (E) |
| Cape Floristic Region | C. angulosa* (E), C. erosa (E), C. geifolia (E), C. mollis (NE), C. platycarpa (NE), C. lobata subsp. lasiocaulis (NE), C. lobata subsp. lobata (NE), C. alchemilloides subsp. alchemilloides (NE) |
| Kamiesberg Centre | C. canescens (NE) |
| Maputaland | C. pinnata (E) |
| Pondoland | C. dryogeton* (E), C. albicans (NE), C. decipiens (NE) |
| Soutpansberg Blouberg | C. erodioides var. tomentosa (E), C. lobata subsp. soutpansbergensis (E), C. cyanomontana* (E) |
| Tanzania: Southern Highlands: Kitulo Plateau | C. foliosa* (E) |
| Zimbabwe: Chimanimani–Nyanga | C. pulchra (E) |
| Centre of endemism . | Species . |
|---|---|
| Angola: Huila: Serra da Chela and surrounds | C. huilensis* (E) |
| Ethiopia: Bale Mts, Simén Mts | C. abyssinica (NE) |
| Madagascar | C. anampoza (E) |
| Malawi: Mzimba District, Lwanjati Hill | C. magnicephala* (E) |
| South Africa: | |
| Albany Centre | C. saxifraga (E), C. vagans* (E); C. lobata subsp. platyptera (E) |
| Barberton Centre | C. ngwenyensis* (E) |
| Cape Floristic Region | C. angulosa* (E), C. erosa (E), C. geifolia (E), C. mollis (NE), C. platycarpa (NE), C. lobata subsp. lasiocaulis (NE), C. lobata subsp. lobata (NE), C. alchemilloides subsp. alchemilloides (NE) |
| Kamiesberg Centre | C. canescens (NE) |
| Maputaland | C. pinnata (E) |
| Pondoland | C. dryogeton* (E), C. albicans (NE), C. decipiens (NE) |
| Soutpansberg Blouberg | C. erodioides var. tomentosa (E), C. lobata subsp. soutpansbergensis (E), C. cyanomontana* (E) |
| Tanzania: Southern Highlands: Kitulo Plateau | C. foliosa* (E) |
| Zimbabwe: Chimanimani–Nyanga | C. pulchra (E) |
Indicates ‘Rarest’ species according to Rabinowitz's criteria of narrow distribution range and habitat specificity and small local populations.
The rarity of each species was assessed using the criteria proposed by Rabinowitz (1981) and Rabinowitz, Cairns & Dillon (1986): geographic range, habitat specificity and local population size. Each of these attributes is dichotomized in an eight-celled block (Table 3). Wide distributions in the geographical range were interpreted as distribution areas with a longest diameter of more than 200 km (Linder, 1995; Schutte, Vlok & Van Wyk, 1995). Habitat specificity refers mainly to soil type (or geological formation) and altitude, where a restricted habitat was coded for a species that occurs on only one soil type (Linder, 1995) and/or has an altitude range of 500 m or less (Schutte et al., 1995). Small populations are those with fewer than 50 individuals; large populations have more than 50 individuals (Linder, 1995). Species were coded as having a large population if somewhere a population of more than 50 individuals is known to exist or is indicated as being, for example, ‘locally abundant’, ‘common’, ‘frequent’ on herbarium labels. Population size was difficult to code for Cineraria as plants tend to grow in patches of suitable habitat, often amongst boulders or rocks (e.g. C. decipiens Harv., C. pulchra Cron and C. austrotransvaalensis Cron), in drainage lines at the base of cliffs (e.g. C. mollis E. Mey. ex DC.) or scattered in the grassland (e.g. C. geraniifolia DC. and C. vagans Hilliard), so best estimates were made based on 18 years of field observations in southern and eastern Africa and notes on herbarium specimens. Population size of the following species (based only on specimen label information and knowledge of the habitat) needs to be confirmed by more current field observations: C. anampoza, C. foliosa O.Hoffm., C. huilensis Cron and C. magnicephala Cron. The term ‘population’ is used here in the sense of groups of potentially interbreeding individuals of a species in close proximity (Barbour, Burk & Pitts, 1980), i.e. the equivalent of IUCN ‘subpopulation’ (IUCN, 2001).
Rabinowitz (1981, 1986) rarity values for the species of pecies of Cineraria
| Geographical distribution . | ||||
|---|---|---|---|---|
| . | Wide . | . | Narrow . | . |
| Habitat specificity . | Broad . | Restricted/narrow . | Broad . | Restricted/narrow . |
| Somewhere large populations | C. abyssinica | C. geifolia (alt)† | ||
| C. albicans | C. pinnata (NT for SA; LC globally) | |||
| C. anampoza | C. saxifraga (geol) | |||
| C. aspera | C. platycarpa (geol) | |||
| C. deltoidea | C. pulchra (geol) | |||
| C. erodioides | ||||
| C. lobata | ||||
| C. lyratiformis | ||||
| C. mollis | ||||
| C. parvifolia | ||||
| C. vallis-pacis | ||||
| All small populations | C. alchemilloides | C. canescens (geol) | C. angulosa[EN B1ab (ii, iii, iv)] | |
| C. austrotransvaalensis (NT)* | C. grandibracteata (alt) | C. atriplicifolia (geol) [VU B1ab(iii)] | ||
| C. decipiens | C. cyanomontana (EN D) | |||
| C. erosa | C. dryogeton (VU D2) | |||
| C. geraniifolia | C. foliosa (DD) | |||
| C. mazoensis | C. glandulosa (VUD2) | |||
| C. huilensis (geol) (DD) | ||||
| C. longipes (VUD2) | ||||
| C. magnicephala (DD) | ||||
| C. ngwenyensis (VUD2) | ||||
| C. vagans[EN B1ab(iii)] | ||||
| Geographical distribution . | ||||
|---|---|---|---|---|
| . | Wide . | . | Narrow . | . |
| Habitat specificity . | Broad . | Restricted/narrow . | Broad . | Restricted/narrow . |
| Somewhere large populations | C. abyssinica | C. geifolia (alt)† | ||
| C. albicans | C. pinnata (NT for SA; LC globally) | |||
| C. anampoza | C. saxifraga (geol) | |||
| C. aspera | C. platycarpa (geol) | |||
| C. deltoidea | C. pulchra (geol) | |||
| C. erodioides | ||||
| C. lobata | ||||
| C. lyratiformis | ||||
| C. mollis | ||||
| C. parvifolia | ||||
| C. vallis-pacis | ||||
| All small populations | C. alchemilloides | C. canescens (geol) | C. angulosa[EN B1ab (ii, iii, iv)] | |
| C. austrotransvaalensis (NT)* | C. grandibracteata (alt) | C. atriplicifolia (geol) [VU B1ab(iii)] | ||
| C. decipiens | C. cyanomontana (EN D) | |||
| C. erosa | C. dryogeton (VU D2) | |||
| C. geraniifolia | C. foliosa (DD) | |||
| C. mazoensis | C. glandulosa (VUD2) | |||
| C. huilensis (geol) (DD) | ||||
| C. longipes (VUD2) | ||||
| C. magnicephala (DD) | ||||
| C. ngwenyensis (VUD2) | ||||
| C. vagans[EN B1ab(iii)] | ||||
IUCN Red Data Categories (in press) are indicated for those species that are not listed Least Concern.
Habitat specialization is indicated as altitudinal (alt) or geological/edaphic (geol) if only one criterion applies.
Rabinowitz (1981, 1986) rarity values for the species of pecies of Cineraria
| Geographical distribution . | ||||
|---|---|---|---|---|
| . | Wide . | . | Narrow . | . |
| Habitat specificity . | Broad . | Restricted/narrow . | Broad . | Restricted/narrow . |
| Somewhere large populations | C. abyssinica | C. geifolia (alt)† | ||
| C. albicans | C. pinnata (NT for SA; LC globally) | |||
| C. anampoza | C. saxifraga (geol) | |||
| C. aspera | C. platycarpa (geol) | |||
| C. deltoidea | C. pulchra (geol) | |||
| C. erodioides | ||||
| C. lobata | ||||
| C. lyratiformis | ||||
| C. mollis | ||||
| C. parvifolia | ||||
| C. vallis-pacis | ||||
| All small populations | C. alchemilloides | C. canescens (geol) | C. angulosa[EN B1ab (ii, iii, iv)] | |
| C. austrotransvaalensis (NT)* | C. grandibracteata (alt) | C. atriplicifolia (geol) [VU B1ab(iii)] | ||
| C. decipiens | C. cyanomontana (EN D) | |||
| C. erosa | C. dryogeton (VU D2) | |||
| C. geraniifolia | C. foliosa (DD) | |||
| C. mazoensis | C. glandulosa (VUD2) | |||
| C. huilensis (geol) (DD) | ||||
| C. longipes (VUD2) | ||||
| C. magnicephala (DD) | ||||
| C. ngwenyensis (VUD2) | ||||
| C. vagans[EN B1ab(iii)] | ||||
| Geographical distribution . | ||||
|---|---|---|---|---|
| . | Wide . | . | Narrow . | . |
| Habitat specificity . | Broad . | Restricted/narrow . | Broad . | Restricted/narrow . |
| Somewhere large populations | C. abyssinica | C. geifolia (alt)† | ||
| C. albicans | C. pinnata (NT for SA; LC globally) | |||
| C. anampoza | C. saxifraga (geol) | |||
| C. aspera | C. platycarpa (geol) | |||
| C. deltoidea | C. pulchra (geol) | |||
| C. erodioides | ||||
| C. lobata | ||||
| C. lyratiformis | ||||
| C. mollis | ||||
| C. parvifolia | ||||
| C. vallis-pacis | ||||
| All small populations | C. alchemilloides | C. canescens (geol) | C. angulosa[EN B1ab (ii, iii, iv)] | |
| C. austrotransvaalensis (NT)* | C. grandibracteata (alt) | C. atriplicifolia (geol) [VU B1ab(iii)] | ||
| C. decipiens | C. cyanomontana (EN D) | |||
| C. erosa | C. dryogeton (VU D2) | |||
| C. geraniifolia | C. foliosa (DD) | |||
| C. mazoensis | C. glandulosa (VUD2) | |||
| C. huilensis (geol) (DD) | ||||
| C. longipes (VUD2) | ||||
| C. magnicephala (DD) | ||||
| C. ngwenyensis (VUD2) | ||||
| C. vagans[EN B1ab(iii)] | ||||
IUCN Red Data Categories (in press) are indicated for those species that are not listed Least Concern.
Habitat specialization is indicated as altitudinal (alt) or geological/edaphic (geol) if only one criterion applies.
The IUCN (2001) Criteria for Red Data listing of species and Guidelines version 6.2 (IUCN, 2006) were used to establish the current conservation status of the species of Cineraria, in consultation with D. Raimondo and L. Agenbag, the Red List Authorities for the region and as published in Raimondo et al. (2009). Global assessments for species outside of South Africa are as given in Cron et al. (2006a).
The ecological traits of distribution, altitudinal range and edaphic selectivity were used as the criteria for determining rarity in this study. The 11 commonest and 11 rarest species in Cineraria were compared in terms of these traits and their overall distribution. The means and standard deviations for distribution range and altitudinal range for each of the subgroups (i.e. ‘Most Rare’ and ‘Common’) were compared against those for all 35 species and two-tailed paired Student'st-tests were performed to test for significant differences (Table 4). Edaphic specialization was compared in terms of frequencies and the Fisher's exact test was used to determine significance for this categorical variable (Table 4).
Comparison of the commonest and the rarest species with all species of Cineraria in terms of distribution range, altitudinal range and edaphic specialization using the two-tailed paired Student'st-test and the Fisher's exact test
| Variable . | Commonest species (N = 11) . | Rarest species (N = 11) . | All species (N = 35) . | Commonest vs. all species statistic, P-value . | Rarest vs. all species statistic, P-value . |
|---|---|---|---|---|---|
| Distribution range: number of grid squares | Mean = 25.7 SD = 19.1 | Mean = 1.7 SD = 0.8 | Mean = 11.9 SD = 14.4 | t = 2.59 *P = 0.013 | t = −2.33 *P = 0.024 |
| Altitudinal range (m) | Mean = 1725.5 SD = 1099.4 | Mean = 410.9 SD = 422.8 | Mean = 989.9 SD = 855.0 | t = 2.36 *P = 0.023 | t = −2.15 *P = 0.036 |
| Edaphic types (0 = one only, 1 = two or more) | Frequencies 0%, 100% respectively | Frequencies 100%, 0% respectively | Frequencies 49%, 51% respectively | Fisher's exact test: *P = 0.0034 | Fisher's exact test: *P = 0.0031 |
| Variable . | Commonest species (N = 11) . | Rarest species (N = 11) . | All species (N = 35) . | Commonest vs. all species statistic, P-value . | Rarest vs. all species statistic, P-value . |
|---|---|---|---|---|---|
| Distribution range: number of grid squares | Mean = 25.7 SD = 19.1 | Mean = 1.7 SD = 0.8 | Mean = 11.9 SD = 14.4 | t = 2.59 *P = 0.013 | t = −2.33 *P = 0.024 |
| Altitudinal range (m) | Mean = 1725.5 SD = 1099.4 | Mean = 410.9 SD = 422.8 | Mean = 989.9 SD = 855.0 | t = 2.36 *P = 0.023 | t = −2.15 *P = 0.036 |
| Edaphic types (0 = one only, 1 = two or more) | Frequencies 0%, 100% respectively | Frequencies 100%, 0% respectively | Frequencies 49%, 51% respectively | Fisher's exact test: *P = 0.0034 | Fisher's exact test: *P = 0.0031 |
Significant at P < 0.05.
Comparison of the commonest and the rarest species with all species of Cineraria in terms of distribution range, altitudinal range and edaphic specialization using the two-tailed paired Student'st-test and the Fisher's exact test
| Variable . | Commonest species (N = 11) . | Rarest species (N = 11) . | All species (N = 35) . | Commonest vs. all species statistic, P-value . | Rarest vs. all species statistic, P-value . |
|---|---|---|---|---|---|
| Distribution range: number of grid squares | Mean = 25.7 SD = 19.1 | Mean = 1.7 SD = 0.8 | Mean = 11.9 SD = 14.4 | t = 2.59 *P = 0.013 | t = −2.33 *P = 0.024 |
| Altitudinal range (m) | Mean = 1725.5 SD = 1099.4 | Mean = 410.9 SD = 422.8 | Mean = 989.9 SD = 855.0 | t = 2.36 *P = 0.023 | t = −2.15 *P = 0.036 |
| Edaphic types (0 = one only, 1 = two or more) | Frequencies 0%, 100% respectively | Frequencies 100%, 0% respectively | Frequencies 49%, 51% respectively | Fisher's exact test: *P = 0.0034 | Fisher's exact test: *P = 0.0031 |
| Variable . | Commonest species (N = 11) . | Rarest species (N = 11) . | All species (N = 35) . | Commonest vs. all species statistic, P-value . | Rarest vs. all species statistic, P-value . |
|---|---|---|---|---|---|
| Distribution range: number of grid squares | Mean = 25.7 SD = 19.1 | Mean = 1.7 SD = 0.8 | Mean = 11.9 SD = 14.4 | t = 2.59 *P = 0.013 | t = −2.33 *P = 0.024 |
| Altitudinal range (m) | Mean = 1725.5 SD = 1099.4 | Mean = 410.9 SD = 422.8 | Mean = 989.9 SD = 855.0 | t = 2.36 *P = 0.023 | t = −2.15 *P = 0.036 |
| Edaphic types (0 = one only, 1 = two or more) | Frequencies 0%, 100% respectively | Frequencies 100%, 0% respectively | Frequencies 49%, 51% respectively | Fisher's exact test: *P = 0.0034 | Fisher's exact test: *P = 0.0031 |
Significant at P < 0.05.
In order to determine if causes for rarity could be determined among life history strategies and/or morphological features, the 11 species in Cineraria exhibiting all three attributes of rarity (i.e. the rarest of the rare species) were compared with the 11 species with no tendency to rarity (a wide distribution range, large populations somewhere and broad habitat requirements). Eleven species-level traits likely to affect survival and longevity or persistence in a habitat (Table 5, variables 1–5) or reproductive output/success (Table 5, variables 6–11) were analysed for significance in relation to rarity. Flowering period and main month of flowering were also investigated, but because rare species are represented by few specimens in herbarium collections, these data were considered insufficient and were not included in the analysis. Where a species was variable for a character (woolly indumentum, cypsela wing breadth and cypsela surface), the most common/frequent state was used in the analysis. Capitulum number and size variables were analysed for the maximum known for the species and the middle of the known range. This is because the maximum often represents an adaptation to extreme environments; for example, populations of a species (e.g. C. deltoidea, C. erodioides) growing at high altitude often have larger and fewer capitula than those at lower altitudes (Cron et al., 2006a, 2007a). Data were gathered from herbarium specimens and field observations. For each morphological feature, at least three measurements per specimen were made when possible and all available specimens of the rare species were measured (see Cron et al., 2006a; Cron, Balkwill & Knox, 2006b for listings) Variable numbers of specimens were measured for the 11 commonest species, ranging from 20 for C. mollis to > 100 for C. deltoidea, C. erodioides and C. lobata L'Hér. (see Cron et al., 2006a, b, 2007a; Cron, Balkwill & Knox, 2007b for listings of specimens).
Species-level traits used to compare 11 commonest and 11 rarest species of Cineraria
| (A) Traits that influence survival and longevity of species resulting in persistence of species in a habitat: |
| 1. Life history: annual or biennial (0); perennial (1) |
| 2. Growth form: herbaceous (0); subshrub to shrub (1) |
| 3. Plant height (m): the maximum plant height for the species |
| 4. Leaf indumentum: long woolly trichomes absent (0); present (1). (May affect evapotranspiration and therefore ability to survive in diverse habitats.) |
| 5. Leaf indumentum: glandular trichomes absent (0); present (1) |
| (B) Traits that influence reproductive output/success (i.e. linked to pollination and seed dispersal) |
| 6. Number of capitula: maximum and middle of range |
| 7. Size of capitula: number of ray florets: maximum and middle of range |
| 8. Size of capitula: number of disc florets: maximum and middle of range |
| 9. Cypsela wing breadth: narrow-winged or margined (0); broad-winged (1). (May influence dispersal by wind.) |
| 10. Cypsela surface: glabrous (0); with mucilaginous duplex trichomes/twin hairs present (1). (May influence seedling establishment.) |
| 11. Cypsela length (maximum; mm) |
| (A) Traits that influence survival and longevity of species resulting in persistence of species in a habitat: |
| 1. Life history: annual or biennial (0); perennial (1) |
| 2. Growth form: herbaceous (0); subshrub to shrub (1) |
| 3. Plant height (m): the maximum plant height for the species |
| 4. Leaf indumentum: long woolly trichomes absent (0); present (1). (May affect evapotranspiration and therefore ability to survive in diverse habitats.) |
| 5. Leaf indumentum: glandular trichomes absent (0); present (1) |
| (B) Traits that influence reproductive output/success (i.e. linked to pollination and seed dispersal) |
| 6. Number of capitula: maximum and middle of range |
| 7. Size of capitula: number of ray florets: maximum and middle of range |
| 8. Size of capitula: number of disc florets: maximum and middle of range |
| 9. Cypsela wing breadth: narrow-winged or margined (0); broad-winged (1). (May influence dispersal by wind.) |
| 10. Cypsela surface: glabrous (0); with mucilaginous duplex trichomes/twin hairs present (1). (May influence seedling establishment.) |
| 11. Cypsela length (maximum; mm) |
Species-level traits used to compare 11 commonest and 11 rarest species of Cineraria
| (A) Traits that influence survival and longevity of species resulting in persistence of species in a habitat: |
| 1. Life history: annual or biennial (0); perennial (1) |
| 2. Growth form: herbaceous (0); subshrub to shrub (1) |
| 3. Plant height (m): the maximum plant height for the species |
| 4. Leaf indumentum: long woolly trichomes absent (0); present (1). (May affect evapotranspiration and therefore ability to survive in diverse habitats.) |
| 5. Leaf indumentum: glandular trichomes absent (0); present (1) |
| (B) Traits that influence reproductive output/success (i.e. linked to pollination and seed dispersal) |
| 6. Number of capitula: maximum and middle of range |
| 7. Size of capitula: number of ray florets: maximum and middle of range |
| 8. Size of capitula: number of disc florets: maximum and middle of range |
| 9. Cypsela wing breadth: narrow-winged or margined (0); broad-winged (1). (May influence dispersal by wind.) |
| 10. Cypsela surface: glabrous (0); with mucilaginous duplex trichomes/twin hairs present (1). (May influence seedling establishment.) |
| 11. Cypsela length (maximum; mm) |
| (A) Traits that influence survival and longevity of species resulting in persistence of species in a habitat: |
| 1. Life history: annual or biennial (0); perennial (1) |
| 2. Growth form: herbaceous (0); subshrub to shrub (1) |
| 3. Plant height (m): the maximum plant height for the species |
| 4. Leaf indumentum: long woolly trichomes absent (0); present (1). (May affect evapotranspiration and therefore ability to survive in diverse habitats.) |
| 5. Leaf indumentum: glandular trichomes absent (0); present (1) |
| (B) Traits that influence reproductive output/success (i.e. linked to pollination and seed dispersal) |
| 6. Number of capitula: maximum and middle of range |
| 7. Size of capitula: number of ray florets: maximum and middle of range |
| 8. Size of capitula: number of disc florets: maximum and middle of range |
| 9. Cypsela wing breadth: narrow-winged or margined (0); broad-winged (1). (May influence dispersal by wind.) |
| 10. Cypsela surface: glabrous (0); with mucilaginous duplex trichomes/twin hairs present (1). (May influence seedling establishment.) |
| 11. Cypsela length (maximum; mm) |
Statistical analyses were computed using Statistica v.6.0 for Windows (Statsoft, 2001). Five morphological traits (plant height, number of capitula, number of ray florets, number of disc florets and cypsela length) were continuous. The other variables were all categorical (Table 5). Two-tailed paired Student's t-tests were used to compare the continuous variables as no a priori hypothesis of the sign of difference between the rare and common species was possible. The Fisher's exact test was used for categorical variables for which frequencies approaching zero were observed (life history, glandular trichomes and cypsela wing breadth). Pearson's χ2-test was applied to the other categorical morphological variables.
RESULTS
Distribution and centres of diversity
Thirty-two (91%) of the 35 species of Cineraria occur in southern and tropical Africa (including southern Angola and Malawi) and 27 (77%) are endemic to southern Africa south of the Kunene River (Fig. 1). Seventeen species occur only in South Africa with another four also occurring in Lesotho; one species (C. decipiens) also occurs in Swaziland and another is endemic to the Ngwenya Hills in Swaziland, bringing to 23 (66%) the number of species in these three southernmost countries of Africa. Only three species (C. alchemilloides DC., C. canescens Wendl. ex Link and C. vallis-pacis Dinter ex Merxm.) occur in Namibia and none is endemic as they also occur in the Northern Cape and/or Western Cape provinces of South Africa. Cineraria pinnata O.Hoffm. ex Schinz occurs in northern KwaZulu-Natal and southern Mozambique.
The centre of diversity for Cineraria is the KwaZulu-Natal Midlands, on the edge of the Drakensberg mountain range (grid squares 2929 and 2930) with eight and nine species, respectively (Fig. 2), with 34% (12/35) of the species occurring in the province as a whole. The Eastern Cape follows as the next most diverse area; grid squares 3126 and 3224 have six species of Cineraria each (Fig. 2).
Only six species of Cineraria occur in Africa north of 16°S (Fig. 3), with few co-occurring (although not necessarily sympatric): C. deltoidea and C. abyssinica co-occur in only four one-degree squares in Ethiopia and C. deltoidea and C. foliosa O.Hoffm. both grow in the southern highlands of Tanzania (Fig. 3). (The distributions of C. abyssinica on the Arabian Peninsula and C. anampoza in Madagascar are not shown here.)
Endemism
Of the six Cineraria species that occur north of 16°S, four are endemic to a specific region/centre of endemism and one is near-endemic (Tables 1 and 2). In Zimbabwe and Mozambique, C. pulchra is endemic to the Chimanimani–Nyanga Centre (Fig. 4), where it occurs in the mist belt at altitudes between 1700 and 2540 m, growing amongst the quartzite rocks (Cron, Balkwill & Knox, 2006c) of the Chimanimani range.
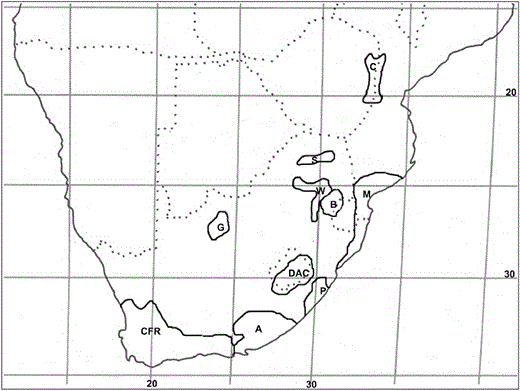
Centres of endemism in southern Africa (sensuVan Wyk & Smith, 2001) with which Cineraria is associated: A, Albany; B, Barberton; C, Chimanimani–Nyanga, CFR, Cape Floristic Region; DAC, Drakensberg Alpine Centre; G, Griqualand West; M, Maputaland; P, Pondoland; S, Soutpansberg; W, Wolkberg.
In southern Africa, 11 species of Cineraria are endemic and six are near-endemic to recognized phytogeographic regions and/or specific mountains, two species are endemic to the KwaZulu-Natal Midlands and two are endemic/near-endemic to Gauteng, both regions not recognized phytogeographically (Tables 1 and 2). Thus, a total of 15 species of Cineraria are endemic to specific regions/mountains in Africa and C. anampoza is endemic to Madagascar (Table 2). At the infraspecific level, four subspecies and a variety are endemic to recognized centres of endemism and three subspecies are near-endemic (Table 2).
Although it is not the centre of diversity, the Cape Floristic Region (CFR, Fig. 4) has the highest number (five) of endemic/near-endemic species and subspecies (three) of Cineraria (Tables 1 and 2). Most of these species are found in the Cape Fold Belt mountains, associated with specific geological formations. Cineraria canescens is a near-endemic to the Kamiesberg Centre of endemism (treated as a separate yet related centre of the CFR by Van Wyk & Smith (2001), where it grows on the massive granitic domes among granite hills.
Cineraria geifolia and C. angulosa, both endemic to the CFR, are unusual in that they occur at sea level: C. geifolia among dune vegetation mainly on the southern coast and C. angulosa on the rocks near Saldanha Bay on the west coast. Cineraria platycarpa DC. is a near-endemic to the Little Karoo Region of the CFR, the arid intermontane valley between the Langeberg and the Swartberg in the Western Cape that extends from about Montagu in the east to Uniondale in the west (Van Wyk & Smith, 2001).
Cineraria saxifraga DC. and C. vagans are endemic to the Albany Centre (Tables 1 and 2; Fig. 4) and it is the region where at least three species (C. erodioides, C. lobata and C. geraniifolia) show a high degree of phenotypic variation, with a subspecies of C. lobata (subsp. platyptera Cron) recognized from the region (Cron et al., 2006a, 2007b).
Cineraria occurs in both the Maputaland and Pondoland Centres of endemism in the Maputaland–Pondoland Region, with C. dryogeton Cron endemic to the Pondoland sandstones and C. pinnata endemic to the Maputaland coastal plains that extend into southern Mozambique. Cineraria albicans N.E.Br. is a near-endemic to the Pondoland sandstones, also occurring at high altitude in the southern Drakensberg in the Eastern Cape. Cineraria grandibracteata Hilliard is near-endemic to the KwaZulu-Natal Midlands as it also occurs on the mountain peaks of the Eastern Cape, but is mainly associated with dolerite.
Cineraria vallis-pacis occurs in the Griqualand West Centre of Endemism in the Northern Cape (Fig. 4). It is unusual among Cineraria spp. as it grows on the north-facing slopes of the low hills in reddish–brown sandy soil derived from the Ghaap Group of the Transvaal Supergroup and mainly in Kalahari Mountain Bushveld, a vegetation type endemic to the centre (Low & Rebelo, 1996). It cannot be considered near-endemic to the region, however, as it also occurs in Highveld Savanna in the mountains around Windhoek in Namibia, where it grows in deep sands along river banks.
In addition, two species are endemic or near-endemic to regions that are not recognized centres of endemism: C. longipes S. Moore is endemic to Gauteng and is unusual in that it occurs on Ventersdorp Basic Lava (basalt), not on the quartzite ridges where C. austrotransvaalensis occurs (Cron et al., 2006a).
Rarity and causes of rarity inCineraria
Eleven species in Cineraria are in the rarest of the rare category (Rabinowitz, 1981) of narrow distribution range, small population size and restricted (or specific) habitat requirements as specified by either soil type/geological formation or altitude or both (Table 3). Many of these rarest species are endemic to a specific region, mountain or centre of endemism, having an extremely limited distribution. Four species occur in only one quarter-degree square and are known from only one locality and three species are known from only two quarter-degree squares (Table 1).
The majority (68.6%) of species have a wide range, although some of them are restricted in habitat or population size. Eleven species are ‘intermediate’ in terms of rarity: they have a fairly wide distribution, but either have small populations and tolerate broader ecological conditions (six species) or have a narrow ecological tolerance, yet somewhere have large populations (five species; Tables 1 and 3). Two species are ‘moderately rare’ in that they have small populations and narrow habitat conditions, yet have a relatively wide distribution range (Tables 1 and 3). Eleven species (34.3%) exhibit no tendency to rarity at all, having wide ranges, occupying diverse habitats and having some large local populations (Table 3). None of the species exhibits the unusual combination of narrow distribution range, broad habitat specificity and either large or small local population size.
The ecological criteria for determining rarity all show significant differences when the ‘commonest’ and ‘rarest’ groups of species are compared with the entire range in all species of Cineraria (Table 4). The eleven rarest species are a homogeneous group with a narrow distribution range (0 = 1.7 ± 0.8 grid squares, Table 4), whereas the commonest species are of a more heterogeneous nature (0 = 25.7 ± 19.1, Table 4), with distributions ranging from six to 62 grid squares (Tables 2 and 4). The groups are less distinct in terms of altitudinal range: the mean of each subgroup clearly shows a difference in range between the subgroups and the entire genus, but the standard deviations from the mean are large (Table 4). Because of the specification of the criteria for rarity in this study, the edaphic specialization is naturally at the extremes for each subgroup; it is significantly different when each is compared with that of all the species (Table 4).
None of the life history strategy, reproductive and vegetative features is significantly different in the 11 commonest species vs. the 11 rarest species of Cineraria (Table 6). There is an equal mix of annual vs. perennial species among both groups and a similar occurrence of growth form, with herbs being slightly more frequent among the rarest species (Table 6). Although there are no significant differences in features linked to reproductive potential, some trends are evident: there is a tendency for some of the rarest species to have fewer and slightly smaller capitula, although, among the 11 commonest species, C. mollis has solitary or paired (relatively large) capitula and C. atriplicifolia DC. (a rare species) may have over 100 (small) capitula per stem. The only three species (C. vallis-pacis, C. lyratiformis and frequently C. erodioides) to have broad wings on their cypselae are among the most abundant and widespread species. Trichomes (twin hairs) are present on the cypselae of 9/11 (81.8%) of the commonest species and only 5/11 (45.5%) of the rarest species (Table 6).
Paired comparisons of commonest and rarest species of Cineraria for 11 variables describing aspects of life history and morphology possibly causally linked to rarity
| Variable . | Common species . | Rarest species . | Statistic, P-value . |
|---|---|---|---|
| 1. Life history (annual/biennial, perennial) | Frequencies 18.2%, 81.8% respectively | Frequencies 18.2%, 81.8% respectively | Fisher's exact test, P = 0.707 |
| 2. Growth form (herb, subshrub/shrub) | Frequencies 45.5%, 54.5% respectively | Frequencies 54.5%, 45.5% respectively | Pearson's χ2-test = 0.18, P = 0.670 |
| 3. Maximum plant height (cm) | Mean (SE) = 75 (9.6) | Mean (SE) = 60.9 (7.3) | Two-tailed paired Student'st-test = 1.17, P = 0.214 |
| 4. Leaf indumentum: long woolly trichomes (absent, present) | Frequencies 54.5%, 45.5% respectively | Frequencies 63.6%, 36.4% respectively | Pearson's χ2-test = 1.91, P = 0.167 |
| 5. Leaf indumentum: glandular trichomes (absent, present) | Frequencies 100%, 0% respectively | Frequencies 81.8%, 18.2% respectively | Fisher's exact test, P = 0.238 |
| 6. Number of capitula per stem (maximum; mid-range) | Mean (SE) = 69.4 (14.8); 39.5 (7.9) | Mean (SE) = 33.9 (16.5); 20.5 (8.9) | Two-tailed paired Student's t-test = 1.597, P = 0.126 (for both max and mid) |
| 7. Number of ray florets per capitulum (maximum; mid-range). | Mean (SE) = 9.9 (0.8); 8.0 (0.5) | Mean (SE) = 9.3 (0.9); 8.0 (0.8) | Two-tailed paired Student's t-test = 0.60; −0.05, P = 0.554; P = 0.962 |
| 8. Number of disc florets per capitulum (maximum; mid-range) | Mean (SE) = 45.7 (5.0); 35.3 (3.7) | Mean (SE) = 36.2 (5.5); 31.1 (5.0) | Two-tailed paired Student's t-test = 1.28; 0.68, P (max) = 0.215; P (mid) = 0.507 |
| 9. Cypsela wing breadth (narrow-winged/margined, broad-winged) | Frequencies 72.7%, 27.3% respectively | Frequencies 100%, 0% respectively | Fisher's exact test, P = 0.107 |
| 10. Cypsela surface: glabrous, twin hairs present | Frequencies 18.2%, 81.8% respectively | Frequencies 54.5%, 45.5% respectively | Pearson's χ2-test = 3.14, P = 0.076/Fisher's exact test, P = 0.091 |
| 11. Cypsela length (maximum) | Mean (SE) = 2.97 (0.16) | Mean (SE) = 2.91 (0.16) | Two-tailed paired Student's t-test = 0.39, P = 0.699 |
| Variable . | Common species . | Rarest species . | Statistic, P-value . |
|---|---|---|---|
| 1. Life history (annual/biennial, perennial) | Frequencies 18.2%, 81.8% respectively | Frequencies 18.2%, 81.8% respectively | Fisher's exact test, P = 0.707 |
| 2. Growth form (herb, subshrub/shrub) | Frequencies 45.5%, 54.5% respectively | Frequencies 54.5%, 45.5% respectively | Pearson's χ2-test = 0.18, P = 0.670 |
| 3. Maximum plant height (cm) | Mean (SE) = 75 (9.6) | Mean (SE) = 60.9 (7.3) | Two-tailed paired Student'st-test = 1.17, P = 0.214 |
| 4. Leaf indumentum: long woolly trichomes (absent, present) | Frequencies 54.5%, 45.5% respectively | Frequencies 63.6%, 36.4% respectively | Pearson's χ2-test = 1.91, P = 0.167 |
| 5. Leaf indumentum: glandular trichomes (absent, present) | Frequencies 100%, 0% respectively | Frequencies 81.8%, 18.2% respectively | Fisher's exact test, P = 0.238 |
| 6. Number of capitula per stem (maximum; mid-range) | Mean (SE) = 69.4 (14.8); 39.5 (7.9) | Mean (SE) = 33.9 (16.5); 20.5 (8.9) | Two-tailed paired Student's t-test = 1.597, P = 0.126 (for both max and mid) |
| 7. Number of ray florets per capitulum (maximum; mid-range). | Mean (SE) = 9.9 (0.8); 8.0 (0.5) | Mean (SE) = 9.3 (0.9); 8.0 (0.8) | Two-tailed paired Student's t-test = 0.60; −0.05, P = 0.554; P = 0.962 |
| 8. Number of disc florets per capitulum (maximum; mid-range) | Mean (SE) = 45.7 (5.0); 35.3 (3.7) | Mean (SE) = 36.2 (5.5); 31.1 (5.0) | Two-tailed paired Student's t-test = 1.28; 0.68, P (max) = 0.215; P (mid) = 0.507 |
| 9. Cypsela wing breadth (narrow-winged/margined, broad-winged) | Frequencies 72.7%, 27.3% respectively | Frequencies 100%, 0% respectively | Fisher's exact test, P = 0.107 |
| 10. Cypsela surface: glabrous, twin hairs present | Frequencies 18.2%, 81.8% respectively | Frequencies 54.5%, 45.5% respectively | Pearson's χ2-test = 3.14, P = 0.076/Fisher's exact test, P = 0.091 |
| 11. Cypsela length (maximum) | Mean (SE) = 2.97 (0.16) | Mean (SE) = 2.91 (0.16) | Two-tailed paired Student's t-test = 0.39, P = 0.699 |
max, maximum; mid, mid-range.
Paired comparisons of commonest and rarest species of Cineraria for 11 variables describing aspects of life history and morphology possibly causally linked to rarity
| Variable . | Common species . | Rarest species . | Statistic, P-value . |
|---|---|---|---|
| 1. Life history (annual/biennial, perennial) | Frequencies 18.2%, 81.8% respectively | Frequencies 18.2%, 81.8% respectively | Fisher's exact test, P = 0.707 |
| 2. Growth form (herb, subshrub/shrub) | Frequencies 45.5%, 54.5% respectively | Frequencies 54.5%, 45.5% respectively | Pearson's χ2-test = 0.18, P = 0.670 |
| 3. Maximum plant height (cm) | Mean (SE) = 75 (9.6) | Mean (SE) = 60.9 (7.3) | Two-tailed paired Student'st-test = 1.17, P = 0.214 |
| 4. Leaf indumentum: long woolly trichomes (absent, present) | Frequencies 54.5%, 45.5% respectively | Frequencies 63.6%, 36.4% respectively | Pearson's χ2-test = 1.91, P = 0.167 |
| 5. Leaf indumentum: glandular trichomes (absent, present) | Frequencies 100%, 0% respectively | Frequencies 81.8%, 18.2% respectively | Fisher's exact test, P = 0.238 |
| 6. Number of capitula per stem (maximum; mid-range) | Mean (SE) = 69.4 (14.8); 39.5 (7.9) | Mean (SE) = 33.9 (16.5); 20.5 (8.9) | Two-tailed paired Student's t-test = 1.597, P = 0.126 (for both max and mid) |
| 7. Number of ray florets per capitulum (maximum; mid-range). | Mean (SE) = 9.9 (0.8); 8.0 (0.5) | Mean (SE) = 9.3 (0.9); 8.0 (0.8) | Two-tailed paired Student's t-test = 0.60; −0.05, P = 0.554; P = 0.962 |
| 8. Number of disc florets per capitulum (maximum; mid-range) | Mean (SE) = 45.7 (5.0); 35.3 (3.7) | Mean (SE) = 36.2 (5.5); 31.1 (5.0) | Two-tailed paired Student's t-test = 1.28; 0.68, P (max) = 0.215; P (mid) = 0.507 |
| 9. Cypsela wing breadth (narrow-winged/margined, broad-winged) | Frequencies 72.7%, 27.3% respectively | Frequencies 100%, 0% respectively | Fisher's exact test, P = 0.107 |
| 10. Cypsela surface: glabrous, twin hairs present | Frequencies 18.2%, 81.8% respectively | Frequencies 54.5%, 45.5% respectively | Pearson's χ2-test = 3.14, P = 0.076/Fisher's exact test, P = 0.091 |
| 11. Cypsela length (maximum) | Mean (SE) = 2.97 (0.16) | Mean (SE) = 2.91 (0.16) | Two-tailed paired Student's t-test = 0.39, P = 0.699 |
| Variable . | Common species . | Rarest species . | Statistic, P-value . |
|---|---|---|---|
| 1. Life history (annual/biennial, perennial) | Frequencies 18.2%, 81.8% respectively | Frequencies 18.2%, 81.8% respectively | Fisher's exact test, P = 0.707 |
| 2. Growth form (herb, subshrub/shrub) | Frequencies 45.5%, 54.5% respectively | Frequencies 54.5%, 45.5% respectively | Pearson's χ2-test = 0.18, P = 0.670 |
| 3. Maximum plant height (cm) | Mean (SE) = 75 (9.6) | Mean (SE) = 60.9 (7.3) | Two-tailed paired Student'st-test = 1.17, P = 0.214 |
| 4. Leaf indumentum: long woolly trichomes (absent, present) | Frequencies 54.5%, 45.5% respectively | Frequencies 63.6%, 36.4% respectively | Pearson's χ2-test = 1.91, P = 0.167 |
| 5. Leaf indumentum: glandular trichomes (absent, present) | Frequencies 100%, 0% respectively | Frequencies 81.8%, 18.2% respectively | Fisher's exact test, P = 0.238 |
| 6. Number of capitula per stem (maximum; mid-range) | Mean (SE) = 69.4 (14.8); 39.5 (7.9) | Mean (SE) = 33.9 (16.5); 20.5 (8.9) | Two-tailed paired Student's t-test = 1.597, P = 0.126 (for both max and mid) |
| 7. Number of ray florets per capitulum (maximum; mid-range). | Mean (SE) = 9.9 (0.8); 8.0 (0.5) | Mean (SE) = 9.3 (0.9); 8.0 (0.8) | Two-tailed paired Student's t-test = 0.60; −0.05, P = 0.554; P = 0.962 |
| 8. Number of disc florets per capitulum (maximum; mid-range) | Mean (SE) = 45.7 (5.0); 35.3 (3.7) | Mean (SE) = 36.2 (5.5); 31.1 (5.0) | Two-tailed paired Student's t-test = 1.28; 0.68, P (max) = 0.215; P (mid) = 0.507 |
| 9. Cypsela wing breadth (narrow-winged/margined, broad-winged) | Frequencies 72.7%, 27.3% respectively | Frequencies 100%, 0% respectively | Fisher's exact test, P = 0.107 |
| 10. Cypsela surface: glabrous, twin hairs present | Frequencies 18.2%, 81.8% respectively | Frequencies 54.5%, 45.5% respectively | Pearson's χ2-test = 3.14, P = 0.076/Fisher's exact test, P = 0.091 |
| 11. Cypsela length (maximum) | Mean (SE) = 2.97 (0.16) | Mean (SE) = 2.91 (0.16) | Two-tailed paired Student's t-test = 0.39, P = 0.699 |
max, maximum; mid, mid-range.
In contrast, all three ecological variables are significantly different (P < 0.0005) between the commonest and rarest species in Cineraria. Thus, restricted range and habitat specificity are strongly indicated as causes of rarity. However, these three attributes are part of the definition of rarity in this study, so this result is not unexpected.
Conservation status
Although 11 species in Cineraria are specific in their habitat requirements and have restricted distributions and population size, causing them to be very rare, only three (C. angulosa, C. cyanomontana Cron and C. vagans) are considered to be endangered in terms of the IUCN Red Data Criteria (Table 1). Five are considered to be vulnerable because of their extremely restricted occurrence, with some occurring in habitats that are threatened. The three remaining species occur in tropical Africa and are considered to be data deficient: C. foliosa is known only from the type locality in southern Tanzania, C. magnicephala only from two sites in Malawi and C. huilensis from the Serra da Chela mountains/plateau in the Huila Province of Angola. Their current status needs to be investigated further in the field. In addition, one species that is rare only in the sense of abundance (C. austrotransvaalensis) is considered to be near threatened (NT), as is one with a restricted habitat if its distribution in South Africa (vs. global, C. pinnata) is considered.
DISCUSSION
Centres of diversity and centres of endemism forCineraria
Plant endemism in southern Africa south of the Kunene, Okavango and Limpopo Rivers is 80% (Cowling & Hilton-Taylor, 1994). This is comparable with what is seen in Cineraria, where 27 of the 35 (77%) species are endemic to the region (Fig. 1, Table 1), if C. pinnata from northern KwaZulu-Natal and southern Mozambique is included.
The centre of diversity for Cineraria, the KwaZulu-Natal Midlands (grid squares 2929 and 2930, Figs 2, 4), is part of the larger Maputaland–Pondoland Phytogeographic Region, although it is not a centre of endemism (Van Wyk & Smith, 2001). It is dissected by river gorges with grasslands predominating, but with pockets of forest on the mountains or hills. The Midlands merge into the foothills of the Drakensberg escarpment and the altitudinal range is considerable (600–1500 m). All these factors contribute to a varied landscape that can accommodate a wide diversity of plants and provides suitable habitat for Cineraria spp. that often have specific requirements.
Cineraria occurs in both minor centres of endemism described in KwaZulu-Natal, the Pondoland Centre associated with the Natal Group and Msikaba Formation sandstones (where about 4% of Asteraceae endemic to KwaZulu-Natal occur) and the Maputaland Centre on the sandy Maputaland coastal plain (Hilliard, 1978; Van Wyk & Smith, 2001). Most endemics in the southern KwaZulu-Natal Drakensberg and Maputaland and Pondoland Centres are herbs or shrubs associated with grasslands (Hilliard & Burtt, 1987; Van Wyk, 1994). Cineraria fits these categories, with most species being perennial herbs or subshrubs. Asteraceae outnumbers any other family of flowering plants in KwaZulu-Natal: there are 551 indigenous species in 113 genera, with 30% endemic to the province (Hilliard, 1978).
Most species of Cineraria are Afromontane in their affinity and are associated with recognized centres of diversity and/or endemism within the Afromontane Centre of Endemism of White (1978, 1983). These montane areas are scattered ‘islands’ of a distinctive flora surrounded by other vegetation types (Beentje et al., 1994). Local endemism is known to be high among grassland herbs throughout the Afromontane region (Matthews, van Wyk & Bredenkamp, 1993; Cowling & Hilton-Taylor, 1997). As noted previously, only C. deltoidea extends almost the full length of the eastern highlands of Africa, where it occurs between 1600 and 4300 m in East Africa and from 200 to 1700 m in KwaZulu-Natal and the Eastern Cape (Cron et al., 2006a, 2007a). Cineraria abyssinica extends from the Afromontane regions of Ethiopia into the Afromontane Regional Centre of Endemism in south-western Arabia, a small region that is floristically extremely impoverished compared with that of Africa (Boulos, Miller & Mill, 1994). In Ethiopia, C. abyssinica and C. deltoidea both occur in the Bale Mountains and C. abyssinica also occurs in the Simén Mountains, both important centres of plant diversity (Beentje et al., 1994). Cineraria deltoidea also grows on the Imatong Mountains in southern Sudan, Mount Elgon and Mount Kenya, all recognized as centres of plant diversity (Beentje et al., 1994).
Three fairly unknown species occur in diverse parts of this Afromontane region of Africa. Cineraria foliosa occurs on the Kitulo Plateau in the Kipengere Range of the southern highlands of Tanzania, a recently proclaimed conservation area renowned for its endemic terrestrial orchids (Davenport & Bytebier, 2004). Cineraria magnicephala is known only from two mountain peaks in central Malawi and C. huilensis is apparently endemic to the Huila region in Angola (Cron et al., 2006a, c), an important centre of endemism (Brenan, 1978) and part of the Afromontane Regional Centre of Endemism (Huntley & Matos, 1994). In addition, some unusual plants possibly related to C. deltoidea occur on Mount Mulanje, another noted centre of diversity and endemism (Beentje et al., 1994).
The Chimanimani–Nyanga Centre, where C. pulchra and C. deltoidea occur, is a meeting ground for Afromontane floristic elements from both north and south and is characterized by high levels of grassland endemism, with most of the endemics being confined to the quartzite-derived soils (Wild, 1964). Affinities of C. pulchra are apparently with C. mazoensis S. Moore, a species that occurs (often associated with granites) further north and west in Zimbabwe and in Malawi and with C. cyanomontana and C. lobata subsp. soutpansbergensis Cron – both endemics from the Soutpansberg Centre to the south (Cron, Balkwill & Knox, 2008).
The Soutpansberg Centre of endemism (Fig. 4) includes the Soutpansberg, a narrow mountain range running east–west, separated in the west from the Blouberg Massif (the highest point, 2051 m) and in the north-east, Lake Fundudzi (Van Wyk & Smith, 2001). Cineraria cyanomontana occurs on quartzite between 1700 and 2000 m on the Blouberg mountain, where fog is common and a fynbos type vegetation is found. Cineraria lobata subsp. soutpansbergensis and C. erodioides var. tomentosa Cron are both endemic to this centre (Tables 1 and 2). Cineraria deltoidea also occurs here, with the nearest populations in the KwaZulu-Natal Midlands to the south and the Chimanimani region to the north, reflecting the floristic links of the wetter parts (mainly southern aspect) of the centre to the north-eastern Drakensberg and the Chimanimani–Nyanga Centres as part of the Afromontane region.
The Barberton Centre of Endemism (Fig. 4), where C. ngwenyensis Cron occurs, is floristically also part of the Afromontane Region and has close links with the Wolkberg Centre (Van Wyk & Smith, 2001), where C. erodioides occurs on quartzites of the Transvaal Drakensberg Escarpment. Most of the endemics are grassland endemics and many are edaphic specialists on serpentine soils, although not many in Asteraceae (Van Wyk & Smith, 2001).
The Drakensberg Alpine Centre (over 1800 m) is recognized as a distinct floristic region by Van Wyk & Smith (2001), based on climatic considerations, not implying floristic links with the Afroalpine regions of east Africa. Although no species of Cineraria is endemic to this centre, ranges of four species extend into it (C. erodioides, C. aspera, C. mollis and C. albicans). Cineraria lyratiformis is also present at lower altitudes. Bolandia pedunculosa (DC.) Cron, one of two known species of Bolandia Cron, the putative sister group to Cineraria (Pelser et al., 2007; Cron et al., 2008), also occurs here.
The Albany Centre has long been recognized as an important centre of species diversity and endemism (Croizat, 1965; Nordenstam, 1969; Beentje et al., 1994; Cowling & Hilton-Taylor, 1994; Phillipson, 1995; Van Wyk & Smith, 2001; Victor & Dold, 2003), with 15% of the approximately 4000 vascular plant species occurring in the region endemic or near-endemic to it (Van Wyk & Smith, 2001). No fewer than five of White's (1983) phytochoria converge in this region, creating a diverse mosaic of floristic elements and vegetation types. It is therefore not surprising that Cineraria has its second highest concentration of species in this region (grid square 3224 and surrounds, Fig. 2), including the endemics C. saxifraga and C. vagans.
Reasons for the high levels of endemism in the Albany Centre include great variation in both topography and geology: it extends from sea level in the south and south-east to c. 2100 m in the north-west and 1500 m in the north-east (Van Wyk & Smith, 2001). In terms of vegetation, it contains almost one-third of Acocks' (1953) 70 veld types (Victor & Dold, 2003).
Within the Cape Floristic Region and centre of endemism, C. canescens (from the Kamiesberg Centre) is similar to and apparently closely related to C. erosa (Thunb.) Willd., which occurs in the mountains near Paarl, Worcester and Montagu. These two species may be evidence of speciation because of isolation resulting from vicariance as the climate became drier, leaving high-altitude refugia in the granitic hills of the Kamiesberg Centre, as suggested by Weimarck (1941).
Rarity inCineraria
Just under one-third of the species in Cineraria (31.4%) are common (widespread), with broad habitat types and somewhere large local populations. The others show varying degrees of rarity because of restriction in habitat type and/or population size (Table 3). The same number (11, 31.4%) of species exhibit all three features associated with rarity: narrow distribution range, restricted habitat specificity and small populations. All eleven very rare species are endemic to a specific region or mountain.
Some of the endemic or near-endemic species have broad distribution ranges, as defined by this study, and two (C. albicans and C. mollis) even fall into the ‘common’ species category despite being near-endemic to the Albany and CFR centres of endemism, respectively. This confirms the point that endemism does not necessarily imply rarity (Kruckeberg & Rabinowitz, 1985; Gaston, 1997). Those species that have a restricted habitat type are all endemic or near-endemics, regardless of local population size. According to Rabinowitz (1981; Rabinowitz et al., 1986), these habitat specialists fall into categories of rarity.
Sparse species (with large ranges and a variety of habitats, but small local population sizes) are as numerous as those with restricted habitats but some large local populations. This is in contrast to the findings of Rabinowitz et al. (1986) in the British and Irish flora, where only 2/177 sampled species were found to be sparse. Rabinowitz et al. (1986) consider these ‘sparse’ species to be the opposite of endemics. Within this category, however, C. erosa is endemic to the CFR and C. austrotransvaalensis is near-endemic to the Gauteng and North-West Provinces. This may reflect differences in interpretation of ‘endemism’ and/or possibly the differences in scale between Britain and Ireland and southern Africa in terms of region or centres of endemism.
Although some species may not be rare, if the infraspecific level (subspecies or varieties) is considered, they are rare entities. For example, C. lobata subsp. lasiocaulis Cron is known from only a small area in the Karoo, C. erodioides var. tomentosa occurs on hills near Gogogo in Venda and C. mazoensis var. graniticola Cron grows only on the granite inselbergs in Zimbabwe.
There has been some debate over the value of recognition of infraspecific categories in taxonomy. Some authors (e.g. Stace, 1976; Snaydon, 1984) considered it useful to recognize variation in species formally, providing more information for conservation of genotypes. Others (e.g. Gaston, 1994) considered that only species can be rare and dismissed lower taxonomic categories. However, what may be considered distinct species by some taxonomists, may by others be differentiated only at the level of subspecies, yet be as genetically distinct. Biosystematic studies are needed to assess the reproductive biology and populations genetics of such species to determine whether the various populations and/or subspecific groupings are interbreeding or are isolated entities that should rather be recognized as distinct species.
This problem could be overcome if populations rather than species could be assessed for conservation purposes, as advocated by Rojas (1992). The concepts of evolutionary significant units (ESUs) and management units (MUs) provide alternatives to the emphasis on species as units for conservation (Ryder, 1986; Waples, 1991; Moritz, 1994; Vogler & Desalle, 1994). An ESU is generally defined as ‘one or a set of conspecific populations with a relatively distinct long-term evolutionary history mostly separate from other such units’ and an MU is ‘one or a set of populations that exchange so few migrants as to be mostly demographically independent of one another at the present’ (Avise, 2005: 86). Consideration of ESUs and MUs thus promotes conservation of genetically distinct and/or demographically autonomous populations within a species, regardless of their rank and the legal implications thereof.
Certainly this is relevant for C. lobata, where the four subspecies have different IUCN assessments and one (C. lobata subsp. platyptera) is considered to be near threatened (Raimondo et al., 2009). It is unlikely that there is currently gene flow among the geographically separated subspecies of C. lobata: C. lobata subsp. lobata in the CFR, C. lobata subsp. platyptera in the Albany Centre; C. lobata subsp. lasiocaulis in the Little Karoo (near Laingsburg) and the Hantam–Roggeveld Centre (near Fraserburg) and C. lobata subsp. soutpansbergensis in the Soutpansberg (Cron et al., 2006a). Recognition of these subspecies as distinct units worthy of conservation because of genetic and ecological distinctness is promoted by their inclusion in the current Red Data listings (Raimondo et al., 2009).
Causes of rarity inCineraria
Most of the narrow endemic or near-endemic species in Cineraria are rare because of specific habitat requirements, either edaphic or altitudinal or both (Tables 1 and 3). The correlation between a narrow distribution range and greater habitat specialization and rarity has been reported elsewhere (e.g. Clarke & Patterson, 2007; Farnsworth, 2007). The role of edaphic factors, in particular, is well documented as a major factor in promoting speciation in the southern African endemic flora (e.g. Cowling, Holmes & Rebelo, 1992; Matthews et al., 1993; Goldblatt, 1997). Endemics in southern Africa are ‘relatively more common on nutritionally peculiar or isolated substrata’ (Cowling & Hilton-Taylor, 1997: 52), an observation true of other areas rich in endemics (e.g. Raven & Axelrod, 1978; Hopper, 1979). The fynbos in the Cape Centre is on nutrient-poor soils derived from ancient quartzites (Cowling & Holmes, 1992), the Pondoland centre is associated with an outcrop of ancient quartzite, part of the Cape Supergroup (van Wyk, 1990, 1994) and the Maputaland grassland endemics are on infertile sandy soils (van Wyk, 1994). Thus, it is not surprising that the species of Cineraria associated with centres of endemism are edaphically specialized and are range restricted.
The restriction in altitudinal range is probably linked to moisture requirements, as many of the species occur in the mist belt (e.g. C. cyanomontana and C. pulchra). This moisture dependency is also reflected in the fact that most Cineraria spp. are found on the southerly or south-easterly aspects of the mountains on which they grow. In addition, many Cineraria spp. grow amongst rocks, which offer some protection against fire in Afromontane grassland and wind and sun in harsh montane or arid environments (e.g. the Kamiesberg or Little Karoo). The run-off from rocks increases the amount of moisture in the soil at the base and, as noted, moisture availability is a critical factor in the survival of Cineraria. This need to grow among rocks in clefts of mountains or at the base of cliffs may have limited the size of local populations of Cineraria as such habitats are, by their nature, limited in availability.
In contrast to Rabinowitz (1981; Rabinowitz, Rapp & Dixon, 1984), Gaston (1994, 1997) considered that only low abundance and/or small range size determine whether a species is rare or not. He argued that applying a third biological parameter (in this instance, habitat breadth) constitutes a prejudgment of the cause of rarity (Gaston, 1997). In this, he followed Reveal (1981), for whom rarity reflected the relatively restricted current status of an extant organism, either in numbers or area, as compared with other organisms of corresponding taxonomic entities. As seen here, the rarest Cineraria spp. all have a narrow altitudinal range and strict edaphic requirements (Table 4). Certainly, consideration of habitat requirement and availability influences or determines how likely a species is to be found in localities not yet accounted for, or how likely it is to move to such areas if a current habitat/location is threatened. Mapping areas of likely occurrence of species based on suitable habitat has resulted in discovering new localities for species (e.g. Pelargonium sidoides DC. in Lesotho, D. Newton, pers. comm.). Consideration of habitat is clearly important for conservation management of a species or suite of species and is of particular value when predictions of effects of climate change are considered.
Life history, reproductive and vegetative features do not apparently strongly influence rarity in Cineraria, although there are some trends among the species that may reflect a causal link or a response to being rare. This is similar to the findings of Farnsworth (2007), where life history and morphological tendencies among the infrequent species were observed, but none was significantly different from the frequent congeneric taxa of coastal sandplain grasslands in north-eastern North America. Of potential interest is the greater prevalence of trichomes (twin hairs) on the cypselae of common species compared with rare species. These twin hairs release mucilaginous threads when wet and are thought to promote their sticking to the substrate once moistened (Roth, 1977). The twin hairs also aid the spread of water over the fruit surface and its subsequent absorption (Hess, 1938; Roth, 1977). Consequently, cypsela morphology could influence dispersal ability, success in anchoring the fruit where it lands and absorption of available water, thereby affecting successful establishment of a seedling.
Phenotypic plasticity is evident among the most widely distributed and abundant species of Cineraria (e.g. C. deltoidea and C. erodioides; Cron et al., 2006a, 2007a) and is possibly an attribute promoting survival in varying habitats and climatic conditions. The reverse of this, i.e. the lack of ability to respond phenotypically to different environments, has been linked to rarity in other members of Asteraceae (McIntyre, 1996). It should be noted, however, that rarity does not always reflect maladaptation (Rabinowitz et al., 1984); it may merely reflect past/current isolation and specialization for a particular habitat or niche.
Phylogenetic position may also be a possible cause of rarity. Recently speciated species may not have dispersed to their full potential (Fiedler, 1986) or a palaeoendemic may have been reduced in range, preserved in often fragmented refugia reflecting past environments. Unfortunately, no robust phylogeny for all Cineraria spp. exists and the molecular (plastid and nuclear) phylogenetic trees for 27/28 (77–80%) of the 35 species provide conflicting information (Cron et al., 2008; G. V. Cron, unpubl. data). Six of the rarest species from remote localities have not yet been included in the phylogenetic analysis. Some of the rare species that have been included (C. pinnata, C. cyanomontana) are placed sister to more widespread species (C. deltoidea, C. lobata, respectively) in the phylogenetic trees based on the internal transcribed spacer (ITS) regions (Cron et al., 2008). A clade containing C. glandulosa Cron, C. ngwenyensis and C. longipes also includes the widespread C. lyratiformis, although relationships within the clade are unresolved (G. V. Cron, unpubl. data). This may indicate recent speciation from a more widespread species as a result of geographic isolation and subsequent habitat specialization. The pattern needs to be confirmed by inclusion of the outstanding rarest species to produce a complete phylogenetic hypothesis, work that is currently in progress.
Implications for conservation prioritization
Twenty-four species of Cineraria are rare (to various degrees) if habitat specialization is recognized as a component of rarity (i.e. Rabinowitz categories of rarity are applied), whereas if only range and abundance are considered, 19 species are rare. Eleven are ‘rarest of the rare’ according to all three parameters: narrow range, low abundance and restricted habitat. Among these species, eight from southern Africa are known to be endangered, vulnerable or threatened and three from tropical Africa are data deficient and require further investigation. This is especially important in areas of Africa previously inaccessible because of civil wars (e.g. C. huilensis in Angola) and/or where collections are a century old (e.g. C. foliosa). For example, habitat destruction is known to have occurred in Angola during the civil war (Huntley & Matos, 1994). In addition, destruction of the rainforests in Madagascar (where C. anampoza occurs) is well documented (e.g. Green & Sussman, 1990). There is a need for urgent assessment of these data-deficient species.
Five of the rare and threatened species in Cineraria (C. cyanomontana, C. foliosa, C. huilensis, C. magnicephala and C. ngwenyensis) occur at high altitude and are therefore likely to be less directly threatened by habitat destruction, more common in the lowlands where agriculture and/or development mainly occur. In addition, some of the mountains where these species occur form part of existing nature reserves (e.g. uKhahlamba Drakensberg Transfrontier Park of KwaZulu-Natal and Lesotho and Ngwenya Hills in Malolotja Nature Reserve, Swaziland) or conservancies (e.g. Blouberg Mountain, Limpopo Province) and the species are therefore currently protected in at least part of their range.
Of great concern, however, are the two coastal rare and threatened species of Cineraria, C. dryogeton and C. angulosa. They are under considerable threat from potential or actual development in the Pondoland region on the east coast and Saldanha Bay region on the west coast of South Africa, respectively. The coastal grasslands of the Pondoland region are currently under threat from a proposed titanium mine, overgrazing, sugar-cane production and commercial timber plantations (Steenkamp et al., 2004). The Saldanha Bay region is greatly threatened by urban development and mining practices (Cordes & Mouton, 1996). Similarly, the grasslands of the Katberg and Amatole mountains of the Eastern Cape where C. vagans occurs are threatened by overgrazing and inappropriate burning regimes. Only 2% of grasslands in South Africa are conserved, whereas 60–80% has been irreversibly transformed (Le Roux, 2002). The recognition of the threat to these rare and threatened species adds to the known threat to these regions and biomes and provides valuable additional data for conservation prioritization and management planning.
Cineraria longipes (VUD2, very rare) and C. austrotransvaalensis (NT, rare in terms of small populations) highlight the need for conservation action in Gauteng where most development in South Africa has occurred and is currently occurring in this densely populated industrial area. The quartzite ridges where C. austrotransvaalensis occurs have been protected from further development in Gauteng by the Ridges Protection Policy, but not in the adjacent North-West Province, where it also occurs.
Knowledge of the rare species (at all three levels of rarity; Table 3) in a genus such as Cineraria, and especially of those that are rare and threatened, can be helpful in future planning for conservation areas and their prioritization. This is especially important with the global climate changes currently known and predicted to occur in southern Africa (Hannah et al., 2002; Pyke, Andelman & Midgley, 2005; Von Maltitz et al., 2006). The likelihood of extinction of some of the rare species in regions that will experience decreasing rainfall and increasing temperature (e.g. the Little Karoo, C. platycarpa) should also be investigated (Rutherford, Powrie & Schulze, 1999; Hannah et al., 2002; Midgley et al., 2003). In addition, once a complete phylogeny for Cineraria is available, it will enable measurements of phylogenetic diversity between the species and contribute towards use of the comparative phylogeographic method for prioritizing conservation areas in southern Africa.
ACKNOWLEDGEMENTS
This work was supported by a National Foundation Research Grant, South Africa, and funding from the University of Witwatersrand Research Committee for one of the authors (GVC). We thank Vivienne Williams and two anonymous referees for useful comments and suggestions that improved the manuscript and the following herbaria for loan of or access to specimens: BM, BOL, BR, COI, E, EA, G-DC, GRA, J, K, LISC, MO, NBG, NH, NU, P, PRE, PRU, S, SAM, SRGH, TCD, UPS, US, WAG and Z.