-
PDF
- Split View
-
Views
-
Cite
Cite
Maxime Gotté, Rajgourab Ghosh, Sophie Bernard, Eric Nguema-Ona, Maïté Vicré-Gibouin, Ikuko Hara-Nishimura, Azeddine Driouich, Methyl Jasmonate Affects Morphology, Number and Activity of Endoplasmic Reticulum Bodies in Raphanus sativus Root Cells, Plant and Cell Physiology, Volume 56, Issue 1, January 2015, Pages 61–72, https://doi.org/10.1093/pcp/pcu141
Close - Share Icon Share
Abstract
The endoplasmic reticulum (ER) bodies are ER-derived structures that are found in Brassicaceae species and thought to play a role in defense. Here, we have investigated the occurrence, distribution and function of ER bodies in root cells of Raphanus sativus using a combination of microscopic and biochemical methods. We have also assessed the response of ER bodies to methyl jasmonate (MeJA), a phytohormone that mediates plant defense against wounding and pathogens. Our results show that (i) ER bodies do occur in different root cell types from the root cap region to the differentiation zone; (ii) they do accumulate a PYK10-like protein similar to the major marker protein of ER bodies that is involved in defense in Arabidopsis thaliana; and (iii) treatment of root cells with MeJA causes a significant increase in the number of ER bodies and the activity of β-glucosidases. More importantly, MeJA was found to induce the formation of very long ER bodies that results from the fusion of small ones, a phenomenon that has not been reported in any other study so far. These findings demonstrate that MeJA impacts the number and morphology of functional ER bodies and stimulates ER body enzyme activities, probably to participate in defense responses of radish root. They also suggest that these structures may provide a defensive system specific to root cells.
Introduction
Plants are able to defend themselves against external threats. When attacked or stressed, plants mobilize their resources in order to fight the enemy, either biotic or abiotic. Such a response can include a perception and a transduction of the danger (Jones and Dangl 2006, Boller and Felix 2009), an induction of an appropriate hormone-related defense response (Verhage et al. 2010), reallocation of energy (Bazzaz et al. 1987, Atkinson and Urwin 2012), expression of defense proteins (van Loon and van Strien 1999), as well as differentiation of cellular organelles (Hayashi and Nishimura 2009).
The endoplasmic reticulum (ER) is the largest intracellular compartment in eukaryotic cells. It performs multiple functions including synthesis and maturation of proteins and lipids, protein storage, maintenance of calcium homeostasis, N-glycosylation and quality control of glycoproteins of the secretory system (Herman and Larkins 1999, Vitale and Denecke 1999, Chrispeels and Herman 2000, Sparkes et al. 2009, Howell 2013). The ER also plays a central role in plant immunity and stress adaptation (Eichmann and Schäfer 2012). One of the notable characteristics of the ER is to form intracellular subdomains or structures that are highly specialized compartments fulfilling specific functions (Staehelin 1997).
Plant cells of the Brassicaceae species have evolved a unique way to mobilize the ER in order to respond to an attack caused by pathogens, insects and wounding. They develop ER-derived fusiform spindle-shaped structures of 5–10 µm in size, termed ER bodies (Hayashi et al. 2001, Matsushima et al. 2003a). These compartments are morphologically distinct from other ER-derived organelles such as the oil bodies or ricinosomes for instance (Shmid et al. 1999). Initially observed in radish (Raphanus sativus) and termed dilated cisternae by Bonnett and Newcomb (1965), ER bodies were later extensively studied and characterized in Arabidopsis thaliana (Hayashi et al. 2001, Yamada et al. 2011, Nakano et al. 2014). They are uniformly distributed throughout the epidermis of cotyledons and hypocotyls, but not found in rosette leaves. They have also been found in roots (Matsushima et al. 2003). PYK10/BGLU23 is a specific and constitutive protein of ER bodies (Matsushima et al. 2003). PYK10 is a β-glucosidase having an ER-retention signal of the KDEL type that has been shown to be a major component of ER bodies in Arabidopsis seedlings (Matsushima et al. 2003). Additional factors such as NAI2, unique to the Brassicaceae family (Yamada et al. 2008), and ERMO3/MVP1/GOLD36 (Nakano et al. 2012) are also ER body-located factors that seem to be required for a proper ER body formation (Yamada et al. 2011).
Formation of ER bodies seems to be regulated by NAI1, a transcription factor specific to the Brassicaceae family (Matsushima et al. 2004). NAI1 regulates the expression of PYK10 and NAI2, which are required for normal ER body formation (Yamada et al. 2008). Indeed, it has been shown that NAI2 deficiency in Arabidopsis reduces the accumulation of PYK10 and leads to abnormal ER bodies and reduction in their number (Yamada et al. 2008).
Several studies have shown that functional ER bodies are required to protect Arabidopsis efficiently against different bioagressors: first, ER bodies were found to accumulate constitutively in epidermal cells of many organs (roots, hypocotyls and cotyledons; Matsushima et al. 2002). They have been shown to fuse with lytic vacuoles when subjected to saline and wound stresses (Hayashi et al. 2001, Matsushima et al. 2002, Matsushima et al. 2003). This finding has suggested that they were probably involved in responses to environmental stresses (Hayashi et al. 2001). Secondly, a net increase in the number of ER bodies is observed in cotyledons and rosette leaves upon wounding or treatment with methyl jasmonate (MeJA) (Matushima et al. 2002), while unwounded/untreated rosette leaves do not form ER bodies (Matsushima et al. 2002). The Arabidopsis coi1 mutant, unable to perceive MeJA, was unable to form ER bodies when treated with this phytohormone. Thirdly, the Arabidopsis nai1 mutant deficient in ER body biogenesis and altered in PYK10 expression developed abnormal ER bodies in rosette leaves in response to an MeJA stimulus (Matsushima et al. 2003, Matsushima et al. 2004). Fourthly, the ML3 protein, a putative MD2-lipopolysaccharide-recognition domain protein, involved in defense signaling, has been found to accumulate in ER bodies and vacuoles (Hakenjos et al. 2013) and ml3 mutants are hypersensitive to herbivore attacks (Fridborg et al. 2013). Finally, nai1 and pyk10 mutants showed an overcolonization by the endophytic fungus Piriformospora indica, resulting in the loss of plant growth promotion (Sherameti et al. 2008). Thus, PYK10/BGLU23 is likely to play a central role in the active defense system against pathogens (Matsushima et al. 2003, Nagano et al. 2008, Sherameti et al. 2008, Ahn et al. 2010, Yamada et al. 2011). PYK10 exhibits both β-glucosidase and β-fucosidase activities required for catalyzing hydrolysis of alkyl-glucosides. Also PYK10 is able to form an active enzymatic complex when it associates with numerous ER bodies and cytoplasmic factors (Yamada et al. 2011). The defense function of these enzymes against pathogens is mostly accomplished by producing toxic compounds as a result of cleavage of glucosinolates by thioglucosidases (Rask et al. 2000, Matsushima et al. 2003, Ahn et al. 2010, Yamada et al. 2011).
The Brassicaceae family (which includes Arabidopsis) encompasses a number of crops of economic interest. It is thus of interest to validate basic research findings (often generated on Arabidopsis) on these economic species in order to improve their resistance to pathogens in a sustainable manner. Radish (R. sativus) is an edible root vegetable of the family of Brassicaceae, grown and consumed throughout the world, and also confronted to many pathogens, one of which is the scab-causing agent, Streptomyces scabiei (Legault et al. 2011). In addition, although ER body-like structures were first observed in radish root cells in 1965 (Bonnett and Newcomb 1965), they were not characterized further and nothing is known about their function so far. It is therefore of interest to characterize ER bodies in radish further and to assess their behavior when challenged with plant hormones involved in activation of defense mechanisms. Here, we have examined the occurrence and distribution of ER bodies in different cell types of R. sativus roots using immunocytochemistry and laser scanning confocal microscopy. We have also characterized the response of ER bodies to MeJA and found that their morphology, number and enzyme activity are significantly modified. Finally, we demonstrate for the first time that MeJA causes ER bodies to fuse together to form very long bodies that have not been observed neither in control radish roots nor in any other plant system studied so far.
Results
Detection and visualization of ER bodies in root cells using anti-PYK10 antibodies
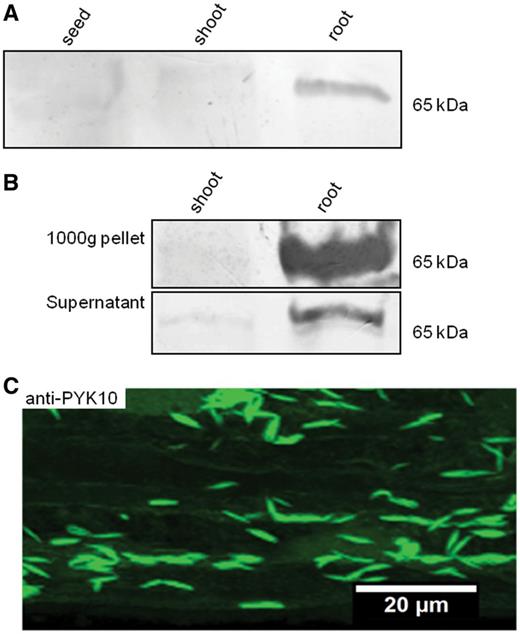
PYK10 has been shown to be a major marker of ER bodies in A. thaliana (Matsushima et al. 2003, Yamada et al. 2011). Therefore, we have taken advantage of anti-PYK10 antibodies to assess the occurrence of ER bodies in radish root cells using Western blotting and immuno-microscopy. Five-day-old seedlings were homogenized in an extraction buffer and the crude extracts obtained were immunoblotted using the anti-PYK10 antibodies. As shown in Fig. 1A, the antibodies cross-reacted with a polypeptide of approximately 65 kDa in the root extract, suggesting that the recognized polypeptide is the radish PYK10, we name here PYK10-like protein. In contrast, no signal was detected either in shoot or in seed protein extracts (Fig. 1A). In Arabidopsis, ER bodies have been shown to accumulate in pellets obtained after a 1,000×g centrifugation of the protein crude extract (Hayashi et al. 2001). Here, the same procedure was applied to a crude extract from radish root and shoot to generate a 1,000 × g pellet fraction. Both the 1,000 × g pellet and supernatant fractions were immunoblotted with the anti-PYK10 antibodies (Fig. 1B). The antibodies reacted strongly with a 65 kDa polypeptide in the 1,000 × g pellet, whereas a weak signal occurred with the 1,000 × g supernatant. In contrast, neither the 1,000 × g pellet nor the supernatant fractions from the shoot contained any detectable PYK10-like protein. These data indicate that a 65 kDa polypeptide reacting with the anti-PYK10 antibodies accumulates in a 1,000 × g pellet of radish root protein extract and is probably located in ER bodies, as previously shown for Arabidopsis (Hayashi et al. 2001).
Occurrence of PYK10-like protein in ER bodies of R. sativus root. (A) Western blot of total protein extracts isolated from R. sativus seeds and 5-day-old shoots and roots probed with anti-PYK10 antibodies. A polypeptide of about 65 kDa is detected using anti-PYK10 antibodies in the root extract but not in seed and shoot extracts. (B) Western blot of the 1,000 × g protein pellet and its corresponding supernatant probed with anti-PYK10 antibodies. Note the strong signal detected in the root 1,000 × g fraction but not in the shoot. A weaker band is detected in the root-derived 1,000 × g supernatant. (C) Immunofluorescent staining of root epidermal cells of R. sativus seedlings using anti-PYK10 antibodies imaged by confocal microscopy. Note the presence of abundant fusiform spindle-shaped structures very similar to those previously described in A. thaliana as ER bodies.
We then checked for the presence of structures recognized by anti-PYK10 within root cells using immunocytochemical labeling and confocal microscopy. As illustrated in Fig. 1C, the images clearly revealed distinguishable fusiform spindle-shaped structures labeled with anti-PYK10 antibodies. The shape and size of the recognized structures resembled those found in Arabidopsis (Hayashi et al. 2001, Matsushima et al. 2003). We have also compared the shape of Arabidopsis ER bodies with those of the spindle-shaped structures observed in radish roots. Roots of an Arabidopsis line expressing a green fluorescent protein [(GFP)–HDEL] (Hayashi et al. 2001, Matsushima et al. 2003) were immunolabeled with anti-PYK10 antibodies coupled to tetramethylrhodamine isothiocyanate (TRITC; Supplementary Fig. S1). Fusiform spindle-shaped ER bodies, similar to those observed in radish roots (see Fig. 1C), were co-labeled with both GFP–HDEL (Supplementary Fig. S1a, Supplementary Data) and anti-PYK10 antibodies (Supplementary Fig. S1b, Supplementary Data). Together immunoblotting and immunofluorescence analyses clearly show that in radish plants, PYK10-like protein is expressed mostly—if not exclusively—in root cells and accumulates in the ER bodies.
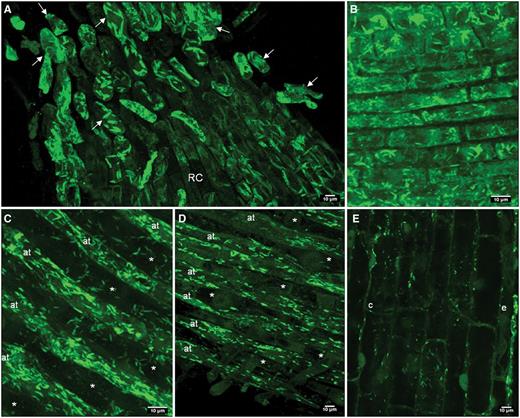
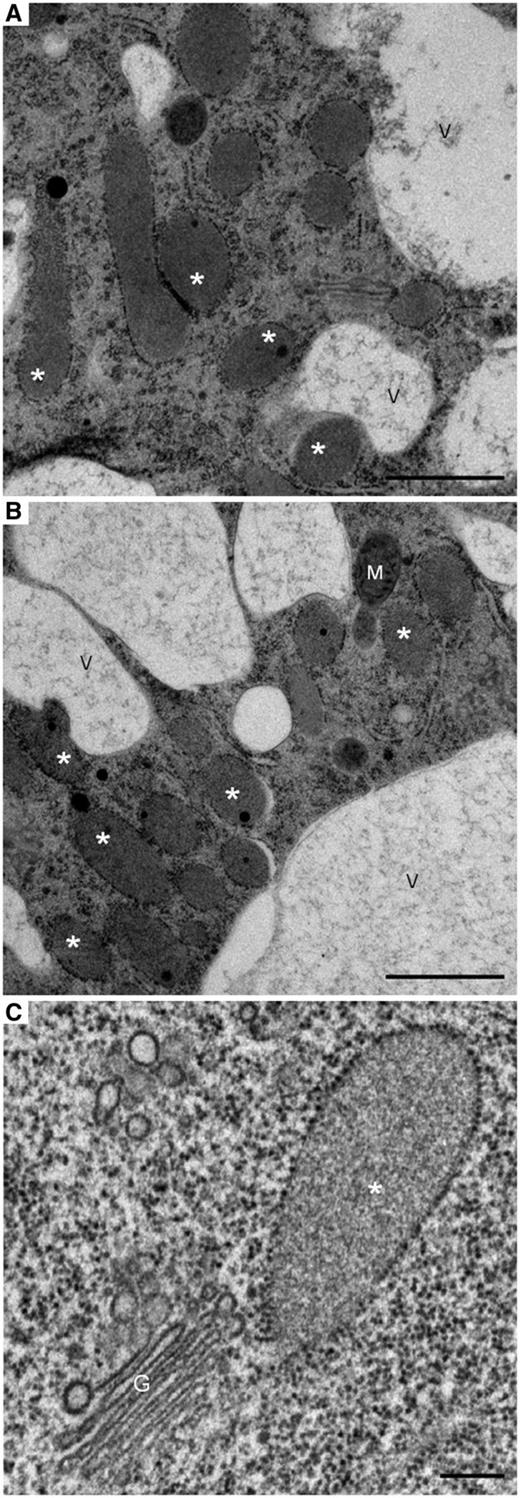
The distribution of ER bodies in radish root tissues was thoroughly investigated using immunofluorescence imaging. ER bodies were visualized by confocal microscopy using anti-PYK10 antibody labeling. As illustrated in Fig. 2, ER bodies were clearly detected in all root zones and cell types investigated including root cap plus border-like cells (Fig. 2A), the meristematic zone (Fig. 2B), the elongation zone (Fig. 2C) and the differentiation zone (Fig. 2D). Epidermal cells along the root tip were principally labeled and easily screened. However, ER bodies were labeled less in trichoblasts than in the atrichoblasts (Fig. 2C, D). Apart from epidermal cells, ER bodies were also present in cortical cells (Fig. 2E). Using electron microscopy on high pressure-frozen root cells, we have been able to visualize ER bodies in different cell types as illustrated in Fig. 3. ER bodies are seen randomly distributed throughout the cytoplasm with no specific location. They were frequently seen in contact with each other, close to the vacuole or even in contact with the vacuole (Fig. 3).
Confocal microscopy images showing ER bodies in different root cell types. ER bodies are stained with anti-PYK10 antibodies coupled with TRITC in the epidermal and cortical cells of different root zones. (A) Border-like cells (arrow) and root cap (RC); (B) meristematic zone; (C) elongation zone; (D) early differentiation zone. Note that ER bodies are hardly detected in trichoblasts (*), whereas they are abundant in atrichoblasts (at). Images A–D are 3D reconstitutions. (E) ER bodies labeled in epidermal (e) and cortical (c) cells of the elongation zone.
Electron micrographs of radish root cells preserved by the high-pressure freezing/freeze substitution technique showing: (A) ER bodies in a meristematic cell. (B) ER bodies in a root cap cell. (C) An ER body close to a Golgi stack in an epidermal cell. Note that some of the ER bodies in (A) are in contact with the vacuole or with each other. Fusion between an ER body and vacuole seems to occur in (B). A and B, scale bars = 1 µm; C, scale bar = 0.2 µm. *, ER bodies; G, Golgi apparatus; M, mitochondrion; V, vacuole.
Taken together, these findings show that radish seedlings express a high level of PYK10-like protein in the roots and that this protein is localized in the ER bodies of various root cell types.
Effect of methyl jasmonate on ER body number and morphology
MeJA is a well-known hormone that mediates wound response, induces resistance against insect/pathogen attack, and regulates the expression of wound-inducible genes (Li et al. 2002). In Arabidopsis, ER bodies have been shown to be induced by MeJA (Matsushima et al. 2002), but the detailed effect on shape has not been investigated.
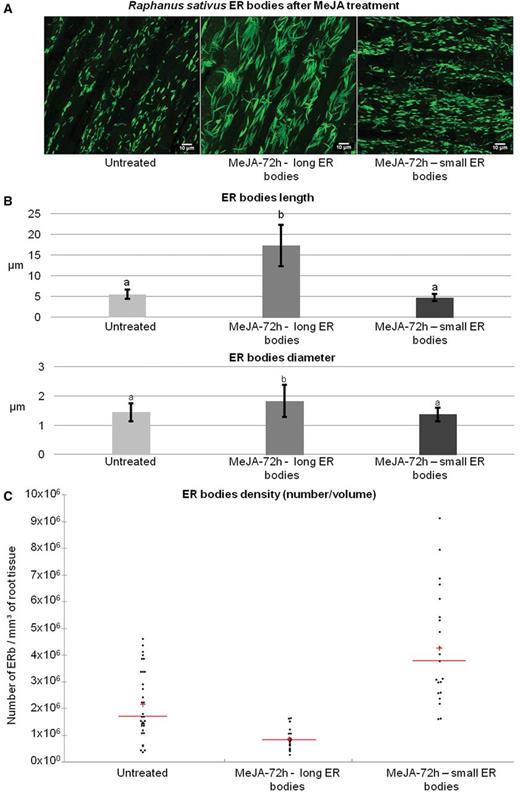
In order to study the effect of MeJA on radish ER bodies, roots were treated with MeJA for 72 h and subsequently immunolabeled with anti-PYK10 antibodies. Initial observations revealed that the 72 h treatment induced two distinct ER body phenotypes as compared with untreated roots: (i) ‘long ER bodies’ and (ii) ‘small ER bodies’ (Fig. 4A; long ER bodies are also shown in Fig. 5).
Effect of MeJA treatment on the morphology and density (number/volume) of ER bodies. ER bodies from the elongation zone of radish roots are labeled with anti-PYK10 antibodies coupled with TRITC and imaged by confocal microscopy. Images are then analyzed by Imaris Bitplane© software. (A) 3D reconstitution images of ER bodies. Untreated plants show normally shaped small ER bodies, while 72 h MeJA-treated plants show two ER body morphotypes: long ER bodies (MeJA-72 h—long ER bodies) and small ER bodies (MeJA-72 h—small ER bodies). (B) Measurement of the length and diameter of ER bodies. Statistical analyses are given in Supplementary Table S1. Error bars show the SE. (C) Scattergram showing the changes in ER body density (number/volume) after MeJA-72 h treatment. 3D reconstitutions were used for counting ER bodies. The results are given as number of ER bodies per mm3 of root tissue, and one dot on the scattergram corresponds to one measurement. Statistical analyses are given in Supplementary Table S1. Red bars, mean; red cross, median.
In order to evaluate these changes accurately, we first examined the treatment effects on the morphology and size of ER bodies. Measurement of mean length and mean diameter of ER bodies showed that in untreated plants, ER bodies had a quite homogeneous morphology with a mean length of 5.50 ± 1.10 µm and a mean diameter of 1.44 ± 0.31 µm (Fig. 4B). It is noteworthy that radish ER bodies are larger than those observed in Arabidopsis cotyledon cells (3.90 ± 0.96 µm) (Nagano et al. 2009) or root cells (4.17 ± 1.14 µm) (Gotté et al. unpublished). We then measured the ER bodies in MeJA-treated root cells. Our analysis reveals that the ER bodies having the first phenotype (long ER bodies) were about three times longer than normal ER bodies, with a mean length of 17.30 ± 4.98 µm and a mean diameter of 1.83 ± 0.54 µm (Figs. 4B, 5). We rarely observed long ER bodies in cells presenting the small ER bodies phenotype, whereas we could observe the presence of small ER bodies in cells in which the long ER bodies are predominant (Fig. 4A). The ER bodies having the second phenotype (small ER bodies) had a length of 4.69 ± 0.86 µm and a diameter of 1.37 ± 0.24 μm (see Supplementary Table S1 for statistical analysis).
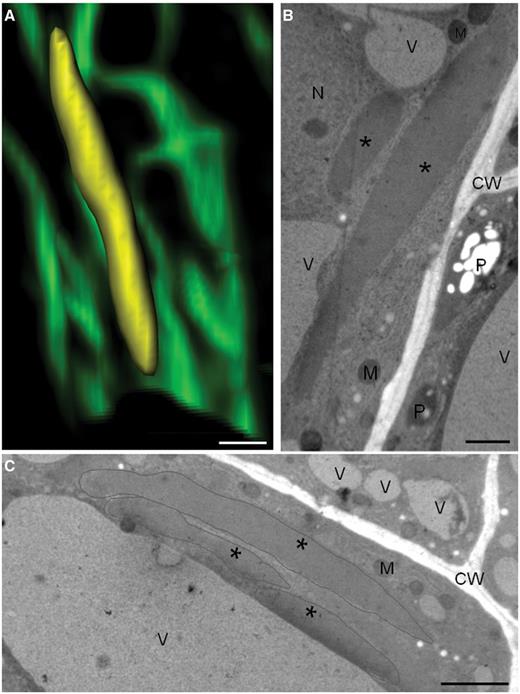
Long ER bodies observed in MeJA-treated cells by confocal and transmission electron microscopy. (A) Confocal micrograph showing a long ER body in three dimensions (reconstruction by Imaris Bitplane© software). The ER body is artificially colored in yellow. (B, C) Electron micrographs of high-pressure freezing/freeze substitution cells showing long ER bodies induced by MeJA treatment. Asterisks mark ER bodies in B–C. CW, cell wall; M, mitochondrion; N, nucleus; P, plastid; V, vacuole. Scale bars: 5 µm in A; 1 µm in B; 2 µm in C.
We then examined the effect of the treatment on the density (number/volume) of ER bodies in the elongation zone. This analysis was carried out on 3D reconstitution images obtained by confocal microscopy, using Imaris Bitplane© software. We first noticed that the density of long ER bodies was significantly lower (9.215 × 105 ± 3.681 × 105 ER bodies mm–3) than that of ER bodies in untreated root cells (2.167 × 106 ± 1.279 × 106 ER bodies mm–3) (Fig. 4C). On the other hand, the density of small ER bodies was significantly higher in treated cells (4.317 × 106 ± 2.109 × 106 ER bodies mm–3) than that of ER bodies in untreated roots and that of the long ER bodies in treated cells (Fig. 4C) (see Supplementary Table S1 for statistical analysis). Moreover, careful examination and counting of ER bodies showed that their density increased from the elongation zone to the meristematic zone, while it decreased in the root cap (Supplementary Fig. S2). The highest increase was observed in the meristematic zone of the root and thus is likely to reflect a role for ER bodies in the protection of the root meristem.
Together, these results demonstrate that MeJA has two distinct effects on ER bodies: in the first case, ER bodies become longer and their number slightly decreases, whereas in the second case the shape of ER bodies does not change much but their density (number/volume) increases considerably. We postulate that elongated ER bodies are formed upon fusion of several small ones, as suggested by the confocal images of these structures being linked to each other by their tips (Fig. 6A). This is also supported by electron microscopy examination of high-pressure frozen and freeze-substituted root cells as illustrated in Fig. 6B, C, D.
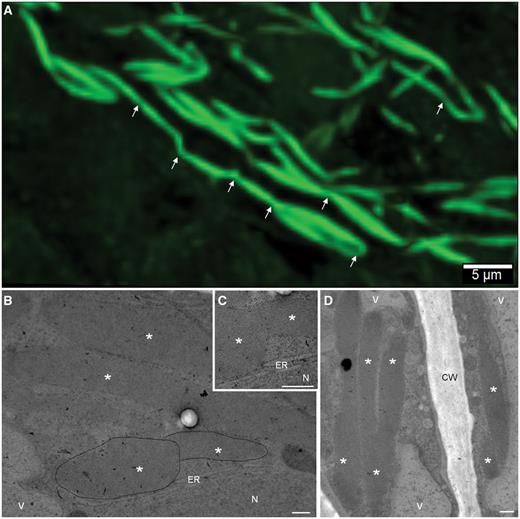
Fusion and link between ER bodies in MeJA-treated root cells imaged by confocal laser scanning (A) and electron microscopy (B–D). Note that several ER bodies are bridged together (in A, arrows indicate the points of contact). The fusion seems to involve the tips of the ER bodies (B–D). *, ER bodies; CW, cell wall; ER, endoplasmic reticulum; N, nucleus; V, vacuole. Scale bars: 5 µm in A; 0.5 µm in B–D.
Effect of methyl jasmonate on PYK10-like protein content
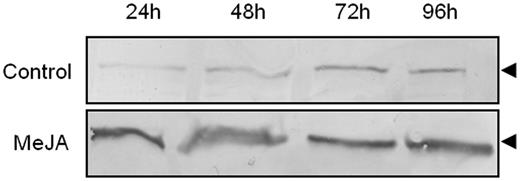
We then studied the effect of MeJA on the level of expression of PYK10-like protein in the ER bodies using immunoblotting with anti-PYK10 antibodies. Five-day-old seedlings were treated with MeJA and the roots were harvested every 24 h during the 4 d following the treatment, and the 1,000 × g pellet known to be enriched in ER bodies was isolated. Western blotting analysis of the ER body-enriched protein extract showed an increase of PYK10-like protein content starting at 24 h after treatment. The expression of the protein was maximum after 2 d of treatment (Fig. 7), which may suggest that the insoluble form of PYK10-like protein increases and reaches a maximum at 48 h, after which it may tend to stabilize to a certain level to attain the maximum level of activity. The data suggest that MeJA is able to stimulate the synthesis and accumulation of PYK10-like protein in the ER bodies.
Effect of MeJA on the expression of PYK10-like protein. Western blot immunodetection of PYK10-like protein in an ER body-enriched fraction (1,000 × g pellet) obtained from untreated or MeJA-treated roots. Note the increased expression of the PYK10-like protein in MeJA-treated samples. The arrowhead indicates the polypeptide recognized by anti-PYK10.
β-Glucosidase and β-fucosidase activities in ER body fractions
The PYK10 gene encodes a β-d-glucosidase (Matsushima et al. 2003) in Arabidopsis. The PYK10/β-d-glucosidase activity is believed to be responsible for the cleavage of glucosinolates, leading to release of toxic molecules necessary for plant defense against fungal pathogens or insects such as scopoletin (Ahn et al. 2010). In addition to glucosidase activity, ER body-enriched fractions of the leaves have been shown to contain β-d-fucosidase activity (Matshushima et al. 2004). Also, PYK10 has been shown to have both activities in A. thaliana (Matsushima et al. 2004).
We have assessed both enzymatic activities in radish roots following treatment with MeJA. Five-day-old seedlings were treated with MeJA and the roots were harvested every 24 h during the 4 d following the treatment, and the 1,000 × g fraction, known to be enriched in ER bodies, was isolated. The fluorogenic substrates 4-methylumbelliferone (4-MU) β-d-glucopyranoside and 4-MU β-d-fucoside were used.
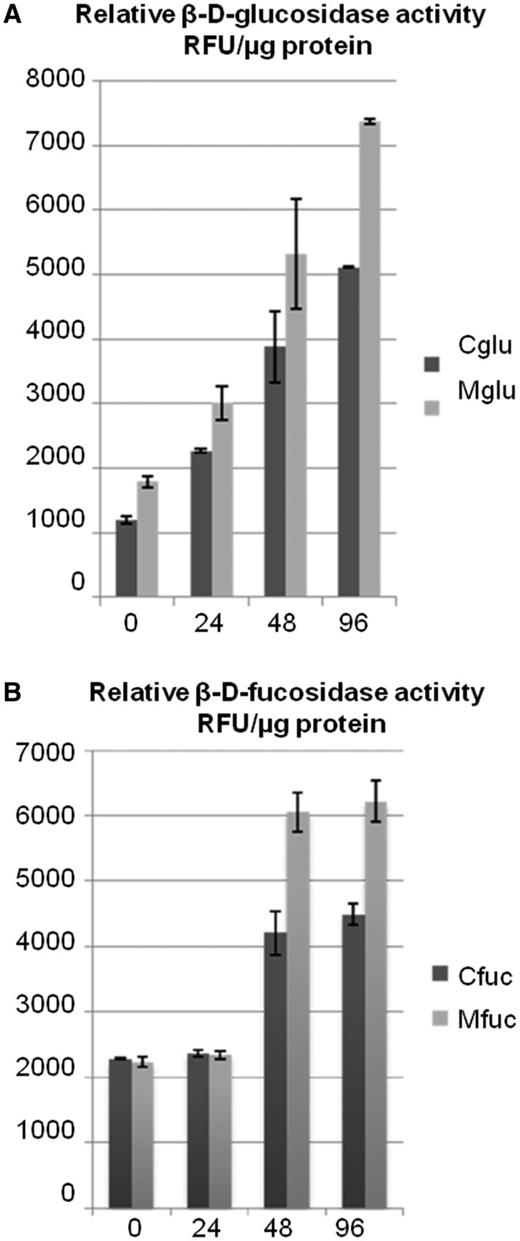
In the presence of MeJA, the β-glucosidase activity increased rapidly and constantly from the onset of treatment, reaching about a 20% increase at 96 h (Fig. 8A). The β-fucosidase activity, on the other hand, seemed to evolve slowly and showed an increase only after 72 h of treatment (Fig. 8B). Together these data demonstrate that the ER body-enriched fractions exhibit both β-d-glucosidase and β-d-fucosidase activities that are most probably linked to PYK10-like protein. In addition, both activities seem to increase significantly in response to MeJA treatment.
Quantification of β-d-glucosidase and β-d-fucosidase activities in MeJA-treated radish roots. (A) β-d-Glucosidase activity in the MeJA-treated plants. The assay was performed using a fluorogenic substrate, 4-MU β-d-glucopyranoside, by monitoring the increase of fluorescence. (B) Fucosidase activity in the MeJA-treated plants with respect to the control. The assay was performed using a fluorogenic substrate, 4-MU β-d-fucosidase, by monitoring the increase of fluorescence. Here the fucosidase activity seems to increase 48 h post-treatment. RFU, relative fluorescence unit; Cglu, β-d-glucosidase activity in the supernatant of control plants; Mglu, β-d-glucosidase activity in the supernatant of MeJA-treated plants; Cfuc, β-d-fucosidase activity in the supernatant of control plants; Mfuc, β-d-fucosidase activity in the supernatant of MeJA-treated plants.
Discussion
The present study shows for the first time that ER bodies of radish root cells can fuse together to form very large bodies in response to the defense-related phytohormone, MeJA. This observation has not been reported in any other plant system studied so far including A. thaliana. The study also shows that functional ER bodies are present in root cells and accumulate PYK10-like protein. So far the role of ER bodies has been studied mostly in Arabidopsis, but little has been reported on these structures in other Brassicaceae species. Arabidopsis studies have clearly established that the β-glucosidase PYK10 is a major protein marker of ER bodies and that these structures are involved in defense (Matsushima et al. 2002, Matsushima et al. 2003, Sherameti et al. 2008, Yamada et al. 2011). Finally, our findings also demonstrate that, in addition to morphology (i.e. size), the number and glycosidase activities of the ER bodies are significantly altered in response to MeJA.
Interestingly, PYK10-enriched ER bodies were predominantly present in radish roots but not in shoots (Fig. 1A, B). Similarly, ER bodies could not be detected in rosette leaves of Arabidopsis (Matsushima et al. 2002). Since BGLU23/PYK10 is a β-d-glucosidase and has toxic end-products, its presence can be attributed to defense against pathogens or other biotic stresses. It may also provide some reasons to explain why roots employ a different mode of defense in comparison with the aerial tissues and that the ER bodies provide a defense system more specific to root cells of radish. Indeed, shoots and roots were found to employ distinct defense machineries against pathogens (Attard et al. 2010, Balmer et al. 2013).
The root is a vital organ for the plant not only as a point of attachment and a feeding organ, but also it interacts closely with the soil, the microbiota and the rhizosphere. In this study, the different zones of the root were investigated for the presence of ER bodies, namely the root cap region, the meristematic zone, and the elongation and differentiation zones. We found that the ER bodies were present in large numbers in all root zones, as revealed by immunocytochemistry using anti-PYK10 antibodies and observed by confocal microscopy (Fig. 2). As well as these regions, we could also visualize ER bodies in border-like cells. Border-like cells are released by root caps of Brassicaceae species (Vicré et al. 2005, Driouich et al. 2007) and their secretions are involved in root protection against various pathogens (Driouich et al. 2010, Driouich et al. 2013). How these ER bodies contribute to the defense system of these cells will await further investigations.
Analyses of the shape of ER bodies of untreated roots indicate that these organelles have a quite uniform morphology. These measurements allowed us to define a standard density (number/volume) and a standard size of ER bodies which are used to compare the ER body phenotypes in MeJA-treated root cells. Indeed, the analyses conducted on the MeJA-treated roots revealed the induction of two distinct ER body phenotypes (Figs. 4, 5): (i) the first type, long ER body, presented a substantial increase of the ER body length and diameter plus a decrease of the ER body number per volume of cell; (ii) the second phenotype, small ER body, presented an ER body shape quite similar to ER bodies from untreated roots whereas their number increased.
We propose that the long ER bodies phenotype is the consequence of fusion of small ER bodies (such as those found in control plants) as supported by confocal and electron microscopic images (see Fig. 6). Such a remarkable change in the size of the ER bodies in response to MeJA in radish has never been reported in any other study on plant ER bodies. The induction by MeJA of such long ER bodies, not seen in other species, is possibly a specific response of radish root cells. Interestingly, ER body fusion with vacuoles has been observed in Arabidopsis cotyledons under abiotic stress (e.g. saline stress conditions) (Hayashi et al. 2001). The fusion and the formation of big ER bodies could be a way to (i) accumulate more PYK10-like protein and other defense enzymes and/or (ii) prepare the plant for a faster release of the ER body content in the case of future attack. Furthermore, we have observed that the density (number/volume) of ER bodies decreases 2-fold when the length increases 3-fold, suggesting that the fusion occurs after the increase in the number of ER bodies. Indeed, MeJA-treated cells appear ‘full’ of long ER bodies covering nearly the entire cytoplasm.
It is noteworthy that the mean length increases by 3.14 times while the mean diameter increases by only 1.3 times; it appears that the ER bodies preserved their fusiform shape after the fusion. ER bodies have frequently been seen linked to each other by their tips. Indeed, careful observation of the images (confocal and transmission electron microscopy data; Fig. 6 A–C) reveals that the fusion involves systematically the tip of the ER bodies, although the contact between two bodies can sometimes occur when they are side by side (Fig 6D). It is also noteworthy that Arabidopsis mutants exhibiting abnormally elongated ER bodies, such as the longer ER body mutant (leb mutant; Nagano et al. 2009), have already been described. Interestingly, the mutated gene in the leb mutant is PYK10. A reduction in the level of NAI2 by RNA interference also induces an elongation of ER bodies in Arabidopsis (Yamada et al. 2008). Similarly to PYK10, NAI2 is an ER body-resident protein which is required for ER body formation. In Arabidopsis, both proteins have been shown to interact with ER body membrane proteins MEB1 and MEB2 to shape the ER bodies (Yamada et al. 2013).These proteins and their interaction are likely to be regulated by MeJA. Further investigation is needed to understand clearly what mechanism is responsible for the fusion and elongation of ER bodies as observed in MeJA-treated radish roots. The interaction between specific proteins from two ER bodies is the more plausible hypothesis since the ER network does not seem to be involved in the fusion. In other cases, the MeJA-treated roots presented a significant increase in the density (number/volume) of ER bodies without any observed change in shape (see Fig. 4). This can be related to the MeJA effect previously described in Arabidopsis (Matsushima et al. 2002). Indeed, ER bodies were observed to be produced in wounded or MeJA-treated rosette leaves, where ER bodies were absent before the treatments. However, a change in morphology and size of ER bodies upon MeJA treatment has never been observed in Arabidopsis seedlings.
Taken together, our results seem to indicate that the MeJA treatment can induce two different phenotypes. Each of them seems to appear with the same frequency, and no specific environmental or experimental condition was suspected to induce one phenotype rather than the other.
The root meristem is a crucial zone of the root where the cells divide rapidly to maintain root growth and development. Our observations clearly show that the highest density of ER bodies is found in the meristematic zone (see Supplementary Fig. S2). It has already been established that ER bodies are involved in the accommodation of the mutualistic fungi P. indica in Arabidopsis roots, and not only PYK10, but probably the entire ER body (or the PYK10 enzymatic complex) plays a role in this plant–microbe interaction (Sherameti et al. 2008). It has been proposed that PYK10, and probably the functional PYK10-associated complex, was directly involved in limiting the entry and colonization of the root by the pathogens, by means of its glycosyl hydrolase activity. Such a mechanism has already been proposed for a gene similar to PYK10, PEN2, encoding a glycosyl hydrolase family 1 (Lipka et al. 2005) and shown to restrict the entry of pathogenic ascomycetes to the roots. Similarly, ER bodies in radish root cells would participate in root protection against pathogens, and the difference in the ER body distribution between the tissues may reflect their defense capabilities. The meristem is a fragile tissue that needs to avoid infection as much as possible, and the high density (number/volume) of ER bodies around the meristem possibly reinforces its protection. It will be of interest to assess the behavior of ER bodies in the presence of Streptomyces scabiei, a root pathogen that causes scab disease in radish.
It is interesting to note that the roots treated with MeJA showed an increase in PYK10-like protein content in a time-dependent manner. The accumulation of this protein with respect to MeJA treatment can be related to the observed alteration of ER body number and size. Such an accumulation could be due to an increase in the number of ER bodies and/or to an increased oligomerization of PYK10-like proteins to form aggregates during fusion of these structures. It can also be due to an increase of the protein content per volume of long ER bodies. In addition to these observations, we have found that the proteins from the ER body-enriched 1,000 × g pellets had a significant amount of β-glucosidase activity which increased significantly over time following MeJA treatment. Similarly, an increase in β-fucosidase activity has also been found. Both activities could be related to PYK10-like accumulation. PYK10 protein has been reported to possess both glucosidase and fucosidase activities in Arabidopsis, and it is believed to act through the hydrolysis of glucosinolates leading to the release of toxic compounds involved in defense against insects and pathogens. Together, these findings suggest that MeJA impacts the number and morphology of the ER bodies and stimulates ER body enzyme activities involved in defense responses of radish root cells.
In summary, the present study provides evidence that functional ER bodies are present in different cell types in radish root and that β-glucosidase PYK10, or a PYK10-like protein, is a marker of these organelles as has been established in A. thaliana. It also shows that ER body formation (i.e. number), morphology (i.e. long ER bodies) and enzyme activities can be regulated by the phytohormone MeJA probably to generate a dynamic and responsive system for defense in radish root against biotic stresses. Future investigations will hopefully shed light on the molecular mechanisms involved of MeJA effects on ER bodies in relation to plant protection.
Materials and Methods
Plant material and growth conditions
Radish (Raphanus sativus var. gaudry) plants were used for all the experiments. Seeds were surface sterilized and sown on half-strength MS medium (Sigma), supplemented with minimal sucrose, and agar (1%) was used as a gelling agent. Plates with seeds were then vernalized for 3 d at 4°C and seeds were allowed to germinate in a photoperiodic chamber under an 18 h daylight period at 22°C for 5–7 d before use.
Chemical treatments
Five-day-old seedlings were used for the chemical treatments. Roots were sprayed with an MeJA solution at a concentration of 50 µM in phosphate buffer (KH2PO4 235 mM and K2HPO4 35 mM, pH 6). The phosphate buffer was used as a control for the ‘untreated’ plants. The excess solution was allowed to drain off and the plates were incubated in the photoperiodic chamber as above, harvested every 24 h for the next 4 d and stored in liquid nitrogen or immunolabeled.
Immuno-cytochemistry: immunofluorescent staining of ER bodies
Plants were fixed for 1 h in 50 mM PIPES pH 7; 1 mM CaCl2; 4% (v/v) paraformaldehyde (PFA); 0.5% (v/v) glutaraldehyde. Plants were washed four times for 10 min each with 50 mM PIPES pH 7; 5 mM EGTA; 2 mM MgSO4 and incubated for 20 min at 25°C with enzymes in 0.01% (w/v) pectolyase Y-23; 0.6% (v/v) pectinase; 1% (v/v) cellulase. Then plants were rinsed three times for 5 min each, with the first rinsing solution plus 10% (v/v) glycerol and 0.5% (v/v) Triton X-100. Plants were incubated in frozen methanol for 15 min at –20°C and rehydrated with three baths of 5 min of phosphate-buffered saline (PBS; 137 mM NaCl; 2.7 mM KCl; 4.3 mM Na2HPO4; 1.5 mM KH2PO4). Aldehydes were saturated with 0.1 M glycine in PBS for 15 min. Plants were saturated with PBS–bovine serum albumin (BSA) 1%. Roots were cut from plants and disposed on 10-well slides and incubated overnight at 4°C with the anti-PYK10 (IM type, Matsushima et al. 2003) antibodies (diluted 1 : 1,000 in PBS–BSA) or with only PBS–BSA (control). After washing with PBS–BSA seven times (5 min each), the slides were incubated for 2.5 h with the secondary antibodies [Sigma anti-rabbit IgG (whole molecule)–TRITC antibody produced in the goat antiserum IgG fraction, buffered aqueous solution (diluted 1 : 50 in PBS–BSA)]. After washing five times for 5 min with PBS–BSA and four times for 5 min with double-distilled water, roots were mounted in anti-fade solution (Citifluor AF2; Agar Scientific), and examined using an inverted confocal laser scanning microscope (Leica TCS SP2 AOBS; objective, ×40 oil; excitation laser, 561 nm; barrier filter, 570–664 nm). Control experiments were performed by omission of the primary antibody.
Treatment of images before analysis
Measurements and counting of ER bodies were performed using Imaris Bitplane© software. Z-stack images were exported to TIFF format. Deconvolution was performed with Autoquant software (MediaCybernetics) using the theoretical pointspread-function (adaptive PSF, 10 iterations). 3D volumes were then further analyzed in Imaris (Bitplane©).
Counting and measurement of ER bodies
ER bodies were counted by adding spots automatically on ER bodies using three filters: quality, intensity center and intensity standard deviation. Each filter was calibrated manually before counting to capture the maximum number of ER bodies. A mean of the values given by these three filters was calculated for each count. More than 40 ER bodies were measured using Imaris Bitplane© software; 36 samples were taken by random sampling.
Electron microscopy
Radish roots (as treated in the ‘Chemical treatments’ section) were prepared by high-pressure freezing and freeze substitution for electron microscopy analysis. Roots were first frozen with HPM100 (Leica Microsystems) in cupule (type B, 16770142, Leica Microsystems) before they were transferred to a freeze substitution automated system (AFS2, Leica Microsystems) pre-cooled to –110°C. Samples were substituted in anhydrous acetone + 2% osmium tetroxide at –90°C for 72 h. The temperature was gradually raised (2°C h–1) to reach –60°C (12 h), –30°C (12 h) and finally 0°C. Then, samples were rinsed with anhydrous acetone (2 × 15 min). Resin infiltration was processed under gentle shaking in a solution containing Spurr/acetone: 25%, 8 h; 50%, 16 h at +4°C; or 75%, 8 h; 100%, 2 × 24 h at room temperature. Polymerization was performed at + 50°C for 24 h. Ultrathin sections (70 nm; ultramicrotome UC6, Leica Microsystems) were collected onto carbon–formvar-coated nickel grids (16708962, Leica Microsystems). Finally, sections were stained for 4 h with 2% osmium tetroxide vapor, followed by 15 min in uranyl acetate (0.5% in methanol) and 15 min in Reynold’s lead citrate. Sections were observed in a Philips, FEI Tecnai 12 Biotwin transmission electron microscope operating at 80 kV, with a CCD camera (ES500W, Gatan).
Preparation of ER body fractions
The seedlings were grown under the above-described conditions and then used for fractionation experiments. The shoot and root sections were severed with a sharp scalpel blade and frozen in liquid nitrogen, and further utilized for the ER-enriched subcellular fraction as described by Matsushima et al. (2003). The samples were crushed in liquid nitrogen and the powder was suspended in HEPES-NaOH (pH 7.5), 0.25 M sucrose and protease inhibitor cocktail (Roche). The suspension was filtered through two layers of Miracloth (Caltech) and was subjected to centrifugation. The clear homogenate was used as a crude extract. The homogenate obtained was centrifuged at 1,000 × g for 30 min at 4°C and the pellet was recovered. The pellet was resuspended in HEPES-NaOH buffer and was used for ultrasonication for 10 min in an ice-cold water bath. The supernatant obtained was further precipitated in ice-cold acetone for 4 h at –20°C. It was further centrifuged at 10,000 × g and the pellet was washed with 80% acetone and air-dried. The resulting pellet was resuspended in the extraction buffer and protein quantity was estimated using the Bradford protein assay Kit (Bio-Rad). About 5–10 µg of protein was used for the SDS–PAGE and immunodetection using PYK10 antibodies.
Immunoblot analysis
After SDS–PAGE, the proteins were transferred electrophoretically onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) using a semi-dry protein transfer apparatus (Transblot, Bio-Rad). The membrane was blocked in 5% non-fat skimmed dry milk for 1 h and later washed three times in Tris-buffered saline (pH 7.5) supplemented with Tween-20 (0.1%). The membrane was incubated for 1 h in PYK10 antibodies and BGLU18 using recommended dilutions of (1 : 30,000, v/v) and (1 : 2,000, v/v), respectively. After incubation, the membrane was washed again as mentioned above and incubated for 1 h in alkaline phosphatase-conjugated secondary antibodies developed in goat against rabbit IgG (Sigma-Aldrich) using a dilution of 1 : 5,000 (v/v). Immunodetection was confirmed with an Alkaline Phosphatase development kit (Promega) following the manufacturer’s instructions. A duplicate gel was always run and stained with Coomassie blue to confirm equal loading of the samples.
Assay of β-d-glucosidase and β-d-fucosidase activities
Total protein extracts were prepared from roots. Six to eight roots were crushed in liquid nitrogen and suspended in 1 ml of 50 mM sodium phosphate, pH 7.0, and centrifuged at 8,000 × g for 5 min. The supernatant was filtered through two layers of Miracloth and further passed through 45 µm filters, and the protein concentration of clear filtrates was determined using the Bradford protein assay reagent. We used 4-MU β-d-glucopyranoside (Sigma) and 4-MU β-d-fucoside (Sigma) as a fluorescent substrate of β-d-glucosidase and β-d-fucosidase, respectively. The filtrates containing 3 µg proteins were incubated in 100 mM sodium phosphate, pH 7.0, for 10 min at 37°C (total 198 µl). A 2 µl aliquot of 100 mM substrate was added to the solution and incubated at 37°C. The fluorescence intensity was measured for kinetic analysis. The fluorescence was monitored using a microplate reader Flexstation 3 (Molecular Devices) using an excitation wavelength of 360 nm and an emission wavelength of 465 nm.
Funding
This work was supported by the University of Rouen; le Grand Réseau de Recherche VASI ‘Végétal-Agronomie-Sols et Innovations’ de Haute-Normandie (France); le Fonds Européen de Développement Régional (FEDER).
Acknowledgments
The authors are grateful to Carole Plasson for her technical assistance and Marie-Christine Kiefer-Meyer for helpful discussion during the course of this work. Thanks are also due to Sofia Driouich for helping with the references.
Disclosures
The authors have no conflicts of interest to declare.
Abbreviations
- BGLU
β-glucosidase
- BSA
bovine serum albumin
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- LEB
long ER body
- MeJA
methyl jasmonate
- 4-MU
4-methylumbelliferone
- PBS
phosphate-buffered saline
- RFU
relative fluorescence unit
- TRITC
tetramethylrhodamine isothiocyante