-
PDF
- Split View
-
Views
-
Cite
Cite
Isao Kobayashi, Shoko Fujiwara, Kosuke Shimogawara, Chiseko Sakuma, Yasuo Shida, Toshikazu Kaise, Hideaki Usuda, Mikio Tsuzuki, High Intracellular Phosphorus Contents Exhibit a Correlation with Arsenate Resistance in Chlamydomonas Mutants, Plant and Cell Physiology, Volume 46, Issue 3, March 2005, Pages 489–496, https://doi.org/10.1093/pcp/pci047
Close - Share Icon Share
Abstract
Pi in the medium relieved the toxicity of arsenate against cellular growth of Chlamydomonasreinhardtii. To investigate the relationship between intracellular P contents and arsenate resistance, we determined the intracellular P contents of arsenate-sensitive and arsenate-resistant mutants, which had been generated by random insertional mutagenesis. All 13 arsenate-resistant mutants showed higher P contents than the parent strain, while arsenate-sensitive mutants with high P contents were not found. In one of the arsenate-resistant mutants, AR3, the intracellular P content was about twice that in the wild type during growth in the absence of arsenate. Arsenate incorporation in AR3 was suppressed within 10 min after the addition of 1 mM arsenate, while Pi incorporation continued even after arsenate uptake ceased. Whereas the P content of the wild type decreased to half in the presence of 0.5 mM arsenate, almost the same degree (about 50%) of decrease was observed in AR3 cells grown in the presence of as much as 3 mM arsenate. AR3, in which PTB1, a homolog of a Pi transporter gene, had been disrupted, exhibited a higher activity of a high-affinity Pi transporter, suggesting that it may be due to a compensatory transport activity. These data suggest that the intracellular level of P is one of the important factors of arsenate resistance.
Introduction
Soil and water in some areas of the world, such as Bangladesh, India and China, are contaminated by arsenic (see Kobayashi et al. 2003). In addition to naturally occurring pollution, arsenic contamination has arisen through human activities, such as mining, industry and waste disposal, all over the world. To solve the problems caused by arsenic pollution, several studies on its toxicity to organisms and on their tolerance of it have been carried out (Sharples et al. 2000, Rosen 2002). Phytoremediation, i.e. removal of arsenic from contaminated water and soil using plants, has also been explored (Pickering et al. 2000, Ma et al. 2001, Ruiz and Romero 2002). However, neither a concrete strategy for protection from arsenic injury nor one for its removal has been established yet. Therefore, it is important to understand the effects of arsenic on various organisms, especially on microalgae, which are primary producers in aquatic ecosystems.
Since the chemical characteristics of arsenate are very similar to those of Pi, arsenate is considered to be taken up via Pi uptake systems. Indeed, several arsenate-resistant (AR) mutants with mutations in Pi uptake systems have been reported. In an arsenate-tolerant strain of the vascular plant Holcus lanatus, the kinetic data for Pi uptake revealed that the activity of a high-affinity Pi uptake system was low (Meharg and Macnair 1992). Pi transport mutants of Saccharomyces cerevisiae, such as a pho87- and pho86-lacking double mutant, and a pho84-lacking mutant, have been reported to be tolerant to arsenate (Bun-ya et al. 1996). In Escherichia coli, there are two Pi uptake systems, Pst, a high-affinity Pi transport system, and Pit, a low-affinity Pi transport system (Rosenberg et al. 1977, Willsky and Malamy 1980). It is known that an arsenate-tolerant strain of E. coli is defective in the Pit system (Rosenberg et al. 1977).
For microalgae, little is known about the mechanisms of arsenate uptake and arsenic resistance. The relationship between intracellular P contents and arsenate resistance has also hardly been investigated. To clarify these problems, we have isolated arsenate-sensitive (AS) and AR mutants of the green alga Chlamydomonas reinhardtii, which have been generated by random insertional mutagenesis. All of 13 AR mutants hardly accumulated arsenic (Fujiwara et al. 2000). In one of the mutants, designated as AR3, PTB1, which is a homolog of the yeast PHO89 gene (a Na+/Pi co-transporter gene) but contains a large insertion, has been disrupted (Kobayashi et al. 2003). In the mutant, arsenate incorporation in a few hours after the addition of arsenate was much lower than in the wild type, while the activity of the high-affinity Pi transport system was higher than in the wild type (Kobayashi et al. 2003). In this study, we measured the intracellular P content of the AR mutant to investigate the relationship between the P content and arsenate resistance.
Results
Intracellular P contents of AR mutants
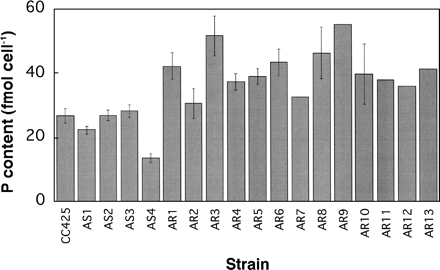
In the series of this research, we isolated AR and AS mutants of C. reinhardtii wall-less strain, CC425, by random insertional mutagenesis, and found that the intracellular arsenic contents in the AR mutants, AR1–AR13, cultured in the presence of arsenate were all lower than that of the parent strain, CC425 (Fujiwara et al. 2000). Some of the AS mutants, namely AS1 and AS3, showed obviously higher arsenic contents than that of the parent strain. We first determined the intracellular P contents of the mutants during exponential growth in the absence of arsenate. All of the AR mutants showed higher P contents than the parent strain (27 ± 2 fmol cell–1). The values were between 31 and 55 fmol cell–1 (corresponding to 10 and 19 µmol mg–1 Chl). On the other hand, AS mutants with high P contents were not found (Fig. 1). The P contents of AS1 and AS4 (22 ± 1 and 14 ± 2 fmol cell–1, respectively) were rather lower than that of the parent strain. These results suggest that intracellular P contents have a reciprocal relationship with intracellular arsenic contents in these mutants, and that both arsenic and P contents may correlate with arsenate resistance. The relationship between P contents and arsenate resistance was investigated further using cell-walled AR3, which has been back-crossed with the wild-type cell-walled strain, CC125.
Pi and arsenate incorporation in the wild type and AR3
Table 1 shows typical results for the initial rates of Pi and arsenate uptake in the wild type and AR3. The initial rate of Pi uptake at 1 mM in the absence of arsenate in AR3 was about three times higher than that in the wild type, while the initial rate of arsenate uptake at 1 mM in the absence of Pi in AR3 was about twice as high as that in the wild type. It is noticed that the arsenate uptake at 1 mM in AR3 was suppressed 10 min after the arsenate addition in both the absence and presence of Pi (1 mM), but not in the wild type (Kobayashi et al. 2003). The arsenate uptake was remarkably inhibited by 10 mM Pi in both strains (Table 1).
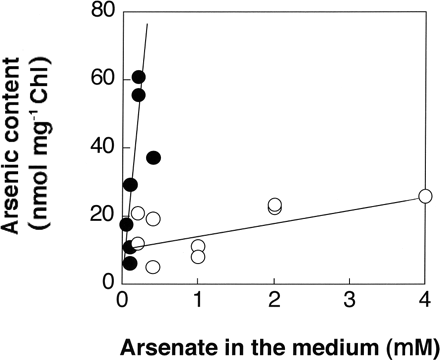
The intracellular arsenic contents of both the wild type and AR3 during the exponential growth in the presence of various concentrations of arsenate were determined (Fig. 2). The intracellular level of arsenic in the wild type increased to about 60 nmol mg–1 Chl with an increase in the arsenate concentration in the medium up to about 0.2 mM. This level may be the critical concentration for survival of the wild type, although some of the arsenate would be converted into organic compounds that are less toxic (Fujiwara et al. 2000). The intracellular arsenic content of AR3 was much lower than that of the wild type, and was only about 25 nmol mg–1 Chl on growth in the presence of 4 mM arsenate.
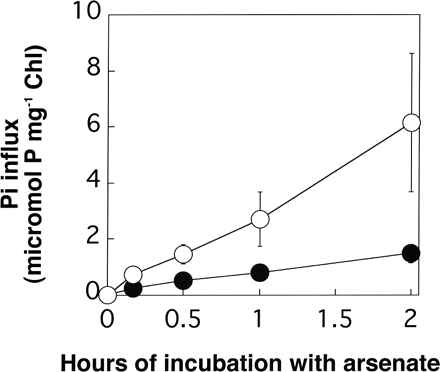
The amount of Pi incorporated into cells that had been incubated in the TAP medium containing 1 mM Pi and 1 mM arsenate was determined (Fig. 3). Pi was incorporated much faster in AR3 than in the wild type over a 2 h time period.
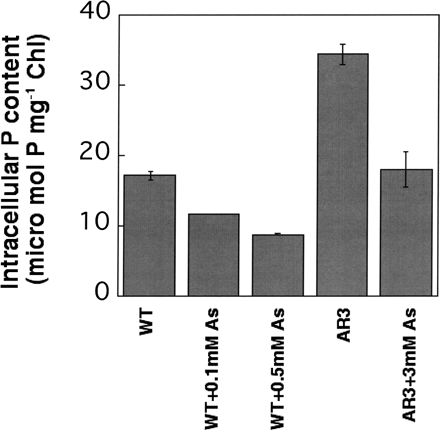
The intracellular P content of AR3 during exponential growth at 1 mM Pi in the absence of arsenate was 34.4 ± 1.0 µmol mg–1 Chl (Fig. 4). This value was about twice that of the wild type (17.2 ± 0.4 µmol mg–1 Chl).
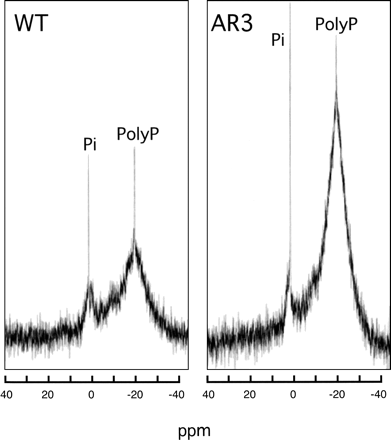
The 31P-nuclear magnetic resonance (NMR) spectra of both the wild type and AR3 mutant showed two signals (Fig. 5): one at the chemical shift of 0.4 ppm, the position of Pi, and the other at that of –22.7 ppm, the position of polyphosphate, although the spectra could not be sharpened using growing cells. Sugar phosphates may be included at the foot of the polyphosphate peak. The result indicates that the major P compounds in Chlamydomonas cells at the logarithmic phase are Pi and polyphosphate. Whether the peak detected in the Pi region was a component in the cytosol or vacuoles could not be determined. In any case, the peaks of both Pi and polyphosphate for AR3 cells were definitely higher than those for the wild type.
Relief of arsenate toxicity by Pi in the wild type and AR3
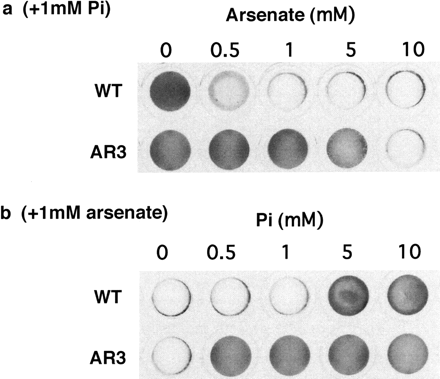
The cell-walled strain of AR3 could grow in TAP medium containing up to 5 mM arsenate, while the wild type could not grow in the presence of >1 mM arsenate (Fig. 6a). When the medium contained 1 mM arsenate, AR3 could grow in the presence of >0.5 mM Pi, whereas the wild type could only grow in the presence of >5 mM Pi (Fig. 6b). This showed that the growth inhibition by arsenate was relieved by the addition of Pi, and that the Pi concentration required for the relief of arsenate toxicity was much less for AR3 than for the wild type.
Inhibition of arsenate uptake by Pi in the wild type and AR3
The effect of Pi on arsenate uptake and that of arsenate on Pi uptake were analyzed further (Table 1). Pi and arsenate uptake were inhibited by arsenate and Pi, respectively, in both strains. In the wild type, 65 and 90% of the arsenate uptake was inhibited by 1 and 10 mM Pi, respectively. The Pi uptake in the wild type was also inhibited by 38 and 57% in the presence of 1 and 10 mM arsenate, respectively. These results support that the two anions are taken up into cells via the same transport system. Furthermore, the rates of Pi uptake were significantly higher than those of arsenate uptake, and the inhibition ratio of arsenate uptake by Pi was higher than that of Pi uptake by arsenate, suggesting that the affinity of the transport system may be higher for Pi than for arsenate. Similar inhibition was also observed in AR3.
The P contents of wild-type cells grown in medium containing 0, 0.1 and 0.5 mM arsenate in the presence of 1 mM Pi were 17.2 ± 0.4, 11.7 ± 0.0 and 8.8 ± 0.0 µmol mg–1 Chl, respectively (Fig. 4). Whereas the P content of the wild-type cells grown in the presence of 0.5 mM arsenate was about half that of those grown without arsenate, the same degree of suppression (about 50%) was observed with AR3 at 3 mM arsenate. These results suggest that arsenate lowered the intracellular P content, but the degree of depression was relieved in the AR cells.
Kinetic analyses of arsenate uptake in the wild type and AR3
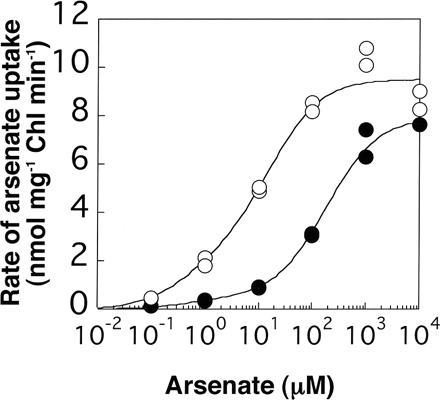
To compare arsenate uptake between the wild type and AR3 more precisely, we obtained concentration dependency curves of initial rates of arsenate uptake (Fig. 7). The initial rate of arsenate uptake in AR3 was significantly higher with relatively low concentrations (10–1–103 µM) of arsenate than that in the wild type. A similar result has been obtained on kinetic analyses of Pi uptake (Kobayashi et al. 2003). It has been found that there are low- and high-affinity Pi transport components in Chlamydomonas (Shimogawara et al. 1999) and that the Vmax of the high-affinity Pi transport component in AR3 is much higher than that of the wild type (Kobayashi et al. 2003). Therefore, the higher arsenate uptake that was observed with low concentrations of arsenate in AR3 was suggested to be due to the higher activity of the high-affinity Pi transport component than that of the wild type.
Expression of genes for Pi transporters in the wild type and AR3
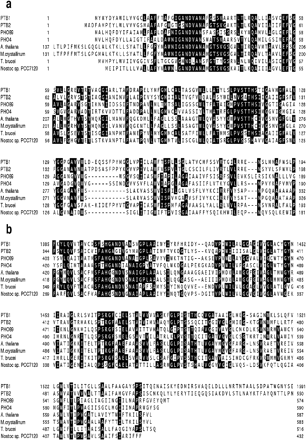
To find a gene for the high-affinity Pi transport component that is activated in AR3, we determined the mRNA levels of putative Pi transporter genes in C. reinhardtii. In AR3, PTB1, a homolog of yeast PHO89 (encoding an Na+/Pi co-transporter), has been disrupted (Kobayashi et al. 2003). During the cloning of PTB1 cDNA, we found another homolog of the Na+/Pi co-transporter gene, PTB2, in C.reinhardtii. The size of PTB2 cDNA is about 2.9 kb and it encodes a putative protein comprising 647 amino acid residues. This protein exhibits significant sequence similarity to the yeast Pho89 protein and other Na+/Pi co-transporters (Fig. 8). While the PTB1 protein (1,666 amino acid residues) carries an additional glutamine- and glycine-rich large hydrophilic region in the middle of its primary structure, the PTB2 protein does not have such an insertional sequence.
Real-time reverse transcription–polymerase chain reaction (RT–PCR) analysis demonstrated that the mRNA level of PTB2 in the wild type at 6 h after P starvation was 184 fmol mg–1 poly(A)+ RNA (the mean for two measurements). Since the level under the P-replete condition was 45 ± 4 fmol mg–1 poly(A)+ RNA (Kobayashi et al. 2003), the expression of PTB2 was revealed to be induced under the P-starved condition in the wild type. On the other hand, the mRNA level of PTB2 in AR3 was 122 ± 17 fmol mg–1 poly(A)+ RNA even in the P-replete condition. An identical trend could be confirmed on Northern blotting (data not shown). These results suggest that PTB2 might encode a derepressible high-affinity Pi transporter.
Discussion
The intracellular P contents of the AR and AS mutants of Chlamydomonas generated by random insertional mutagenesis were investigated. Interestingly, all the AR mutants obtained showed higher P contents than the parent strain, while some AS mutants (AS1 and AS4) have lower P contents (Fig. 1). Since all the AR mutants showed much lower arsenic contents than the parent strain, and AS1 and AS3 had higher arsenic contents (Fujiwara et al. 2000), the intracellular arsenic/P ratios are lower in the AR mutants, and higher in some of the AS mutants than in the parent strain.
In AR3, the rate of Pi uptake was higher than in the wild type (Fig. 3), and that of arsenate uptake was also higher immediately after the addition of arsenate, but decreased rapidly and almost stopped within 10 min (Kobayashi et al. 2003). As a result, the intracellular arsenic content in AR3 cells grown in the presence of arsenate was much lower than in the wild type (Fig. 2). Since the release of arsenic was not accelerated in AR3 (Kobayashi et al. 2003), the low arsenic content was considered to be due to suppression of the arsenate uptake. On the other hand, the intracellular P content in AR3 was higher in both the presence and absence of arsenate than in the wild type (Fig. 4). Kinetic analyses suggest that AR3 exhibit a higher activity of a high-affinity Pi transporter than the wild type (Kobayashi et al. 2003). These suggest that in AR3, in which PTB1, a homolog of a Pi transporter gene, had been disrupted (Kobayashi et al. 2003), the activity of the high-affinity Pi transporter is increased by a compensatory transport activity, and thereby AR3 shows a higher P content. AR3 also shows a higher activity of a high-affinity arsenate transporter initially, but the activity was suppressed immediately after the addition of arsenate. The mechanism of suppression is still unclear. However, the high-affinity Pi/arsenate transporter(s) might be encoded by any of PTB1 homologs, e.g. PTB2, as expected from the expression patterns in the wild type and AR3.
In general, there are two types of arsenate resistance mechanisms in organisms: (i) the intracellular arsenic concentration is somehow kept low in spite of the presence of arsenate outside of the cells; and (ii) the toxicity of arsenate is reduced by improved acclimation and/or enhanced detoxification. Examples of case (ii) include the conversion of inorganic arsenic into organic arsenic compounds (Kaise et al. 1988) and the chelation of inorganic arsenic by SH compounds (Cobbett and Goldsbrough 2002). The former mechanism also seems to operate in Chlamydomonas, since an AS mutant (AS2) that cannot convert arsenate to dimethylarsinic acid quickly has been isolated (Fujiwara et al. 2000). The mechanism may be a relatively time-consuming one since the accumulation of dimethylarsinic acid is not evident until >24 h after exposure to arsenate in Chlamydomonas. As for the latter mechanism, phytochelatins are considered to be involved in detoxification of not only heavy metals but also arsenic in many vascular plants (Maitani et al. 1996, Ha et al. 1999, Schmöger et al. 2000, Hartley-Whitaker et al. 2001). However, phytochelatin biosynthesis was scarcely stimulated by arsenate in the wild type of Chlamydomonas (I. Kobayashi, unpublished data). For case (i), the low intracellular arsenic concentration is caused by the suppression of arsenate influx or the stimulation of arsenic efflux. This is probably important for the initial resistance immediately after exposure. Several AR mutants with reduced activities of both arsenate and Pi uptake have been reported in various organisms, as described in the Introduction (Rosenberg et al. 1977, Meharg and Macnair 1992, Bun-ya et al. 1996). The arsenate resistance of the AR3 mutant could also be explained by the suppression of arsenate influx in case (i). However, AR3 seems to have a new type of arsenate resistance mechanism, since it exhibits rather higher activity of Pi uptake than the wild type. The high intracellular P content is supposed to contribute to the relief of the arsenate toxicity, as suggested in an AR mutant of Arabidopsis (Lee et al. 2003).
The mutant of Arabidopsis also has a unique mechanism of arsenate resistance: although it accumulates levels of arsenic similar to that accumulated by the wild type, it shows increased Pi uptake. On the other hand, AR3 has a new mechanism which is different from that of other AR mutants including the Arabidopsis mutant: it shows unique characteristics of low accumulation of arsenic and high accumulation of P. Although the mechanism of alteration of Pi/arsenate transport activity in AR3 has not been elucidated yet, the gene responsible for the AR3 mutation, PTB1, has been identified (Kobayashi et al. 2003). PTB1 exhibits significant sequence similarity to the Na+/Pi co-transporter of yeast (Pho89), but it contains a large insertion in the middle of its primary structure. The result of the complementation test suggested that PTB1 is a factor involved in arsenate (or Pi) uptake. Since the mRNA level of PTB1 was quite low, PTB1 may be not a Pi transporter but rather a regulation factor of Pi uptake (Kobayashi et al. 2003). However, any information about abundance of the PTB1 protein, its stability or its localization has not been obtained, and consequently it cannot be ruled out that PTB1 may transport Pi or arsenate to a specific cellular compartment. The function of PTB1 remains to be analyzed.
In AR3, PTB2, which is a PTB1 homolog but does not contain such an insertion in the middle part, was highly expressed. The increased activity of a high-affinity Pi transport component observed in AR3 (Kobayashi et al. 2003) might be the result of compensatory transport activity(ies) of PTB1 homolog such as PTB2. However, we could not elucidate the exact function of PTB2, because PTB2 did not have the activity of Pi transport in complementation tests using the PAM2 yeast mutant (pho84, pho89) lacking two high-affinity Pi transporters. At present, we could conclude that PTB1 homolog(s) including PTB2 might encode derepressible high-affinity Pi transporter(s), and that the gene product(s) could be involved in the Pi transport component(s) that are activated in AR3.
Recently, the Chlamydomonas genome project has advanced, and the database search has suggested that the Chlamydomonas genome has at least 10 genes of yeast pho89 homologs including PTB1 and PTB2. To date, we have confirmed by RT–PCR that at least nine of them are expressed in the wild-type cells (S. Fujiwara and K. Shimogawara, unpublished data). Analyses of the expression patterns, intracellular localization and kinetics of Pi and arsenate uptake in gene-disrupted cells will reveal the functions of the genes. Several genes including PTB2 might encode high-affinity Pi/arsenate transporters. In summary, a high affinity of the Pi/arsenate transport system for Pi and a high intracellular P content, which are presumably due to high activity(ies) of Pi/arsenate transporter(s) encoded by some gene(s) belonging to the PTB multigene family, may be a reason for arsenate resistance in AR3.
Materials and Methods
Algal cells and culture conditions
Chlamydomonas reinhardtii CC125 (wild type mt+), and AS and AR mutants, which were obtained by random insertional mutagenesis (Fujiwara et al. 2000), were grown mixotrophically in TAP medium (containing 1 mM Pi) (Harris 1989). Cultures in flasks were agitated on a gyratory shaker (120 rpm) at 27°C under continuous illumination at 80 µmol photons m–2 s–1 with fluorescent tubes. Before physiological analyses, an AR mutant, AR3, was back-crossed four times with the wild type to ensure that the phenotype was the result of a single lesion in a homogeneous genetic background.
Arsenate and Pi uptake
Log phase cells grown in TAP medium were harvested by centrifugation, washed with the Pi-free TAP medium twice, and then suspended with the Pi-free TAP medium. After the pre-incubation for 20 min, appropriate concentrations of arsenate and Pi were added to the cell suspension. The pre-incubation and incubation were both performed at 27°C under continuous illumination at 80 µmol photons m–2 s–1.
Arsenate uptake at 1 mM in the absence or presence of various concentrations of Pi was measured for 5 min, during which time arsenate uptake was linear. Arsenate and Pi were added at the same time. Arsenate uptake at appropriate concentrations in the absence of Pi was also determined. Arsenate taken up by the cells was measured with an atomic absorption spectrophotometer (Spectra AA220; Varian, Australia), as described previously (Fujiwara et al. 2000).
Pi uptake at 1 mM was also measured for 5 min in the absence or presence of various concentrations of arsenate. Pi and arsenate were added at the same time. Pi taken up by the cells was determined by quantification of the radioactivity of 32Pi (specific radioactivity, 7 kBq nmol–1).
Both arsenate and Pi uptake were stopped by adding a 2-fold volume of ice-cold washing buffer (TAP enriched with 20 mM KH2PO4 and 0.005% Tween-20, pH adjusted to 5.0 with Pi). We tested arsenate and Pi uptake after the addition of the washing buffer and ensured that both uptakes were inhibited by such a high concentration of Pi under low pH conditions.
Measurement of Pi and arsenate incorporated into cells in the presence of 1 mM Pi and 1 mM arsenate
Log phase cells grown in TAP medium were harvested by centrifugation, and then washed twice and suspended with the Pi-free TAP medium. After the pre-incubation for 20 min, arsenate (1 mM) and 32Pi (1 mM, specific radioactivity, 7 kBq nmol–1) were added to the cell suspension. The pre-incubation and incubation were both performed at 27°C under continuous illumination at 80 µmol photons m–2 s–1. At appropriate intervals, the cells were precipitated and washed four times with the ice-cold washing buffer. 32Pi and arsenate incorporated into the cells were quantified as described above.
Determination of intracellular arsenic and phosphorus contents
Cells were grown to the logarithmic phase for several days in TAP medium containing various concentrations of arsenate. Cells were harvested and washed four times with the ice-cold washing buffer. The total arsenic content of cells was measured as described above.
For determination of intracellular P concentrations, cells were cultured in the normal TAP medium or TAP medium for which the Pi concentration was reduced to 0.1 mM (TAP-0.1 mM Pi medium) containing 32Pi (specific radioactivity, 0.7 kBq nmol–1) to the logarithmic phase for several days. P incorporated into the cells was quantified as described above.
31P-NMR measurements
Cells at the logarithmic phase were washed with 10 mM Tris-MES buffer, pH 7.0. The resulting cell suspension, which was concentrated to the density of 5 (mg Chl) ml–1, was transferred to a sample tube (diameter, 5 mm). 31P-NMR spectra were measured with a Bruker PDX400 NMR spectrometer, and a 5 mm diameter QNP probe head operating at 162.2 MHz in the pulsed Fourier transform mode without proton decoupling and unlocked at room temperature. A total of 1,024 scans were accumulated for each spectrum with line broadening of 5.0 Hz. Identification of the form of Pi was performed by means of the assignments reported by Yang et al. (1993). Chemical shifts were measured in ppm relative to external 85% Pi (w/w).
Real-time RT–PCR
A 100 µg aliquot of total RNA was obtained from 1×107 cells by means of an RNeasy Mini kit (QIAGEN, CA, U.S.A.). A 2 µg aliquot of poly(A)+ RNA was purified from 100 µg of total RNA using Dynabeads Oligo (dT)25 (Dynal, Norway). cDNA was synthesized from 100 ng of poly(A)+ RNA with ReverTra Ace (Toyobo, Osaka, Japan). Real-time RT–PCR was performed with a LightCycler (Roche, Tokyo, Japan), using PTB2-cDNA specific primers, 5′-GGTGCCAAGACCCTCACACTGA-3′ and 5′-AGCCGATGATTGGCACCAATGAT-3′. The reaction mixtures comprised 2 mM Mg2+, 0.2 mM each deoxynucleotide, 0.25 µM each primer and 0.4 U of KOD plus Taq polymerase (Toyobo, Osaka, Japan). The PCR cycle used was as follows: 98°C (10 s), 68°C (5 s), 72°C (10 s). PCR products amplified with specific primers were used as quantification standards at concentrations from 0.1 fM to 1 pM. The optimum cycle number for real-time PCR was between 14 and 30 cycles. The amount of mRNA was calculated from the cycle number when the fluorescence on PCR amplification reached a definite level. We confirmed that the amount of PCR product increased with an increasing number of cycles and that the reaction components were not limiting. The amount of mRNA was normalized by being divided by the amount of poly(A)+ RNA used for the reaction. The poly(A)+ RNA was quantified by measuring the A260.
Acknowledgments
This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture, Japan (13640657, 13740463 and 13874112), the Promotion and Mutual Aid Corporation for Private Schools, CREST of JST to M.T. and S.F., and a Sasakawa Scientific Research Grant from The Japan Science Society to I.K.
Present address: Insect Biology and Sericology Department, National Institute of Agrobiological Sciences, Owashi, Tsukuba, Ibaraki, 305–8634 Japan.
Fig. 1 Intracellular P contents in various AS and AR mutants, and the parent strain (CC425). Cells were cultured in 32Pi-containing TAP-0.1 mM Pi medium to the logarithmic phase for several days. 32P incorporated into the cells was quantified. The data are the means ± SE for 3–13 independent experiments, except for five strains. For the five strains, SE cannot be indicated, since the data are from only one or two experiments.
Fig. 2 Arsenic incorporated into wild-type (filled circles) and AR3 (open circles) cells. Cells were grown to the logarithmic phase for several days in TAP medium containing 1 mM Pi and various concentrations of arsenate (0.05, 0.1, 0.2 and 0.4 mM for the wild type; 0.2, 0.4, 1, 2 and 4 mM for AR3). The data obtained by two independent experiments are plotted.
Fig. 3 Time courses of Pi incorporation into the wild type (filled circles) and AR3 (open circles) during incubation in arsenate-containing TAP medium. Log phase cells grown in TAP medium were harvested by centrifugation, and then washed and suspended with the Pi-free TAP medium. After the incubation for 20 min, arsenate (1 mM) and Pi (1 mM) were added to the cell suspension. Cells were harvested at appropriate intervals and Pi incorporated into the cells was determined by quantification of radioactivity from tracer amounts of 32Pi. The data are the means ± SE for three independent experiments.
Fig. 4 Intracellular P contents in the wild type and AR3 grown in TAP medium with or without arsenate. Cells were cultured in 32Pi-containing medium to the logarithmic phase for several days. 32P incorporated into the cells was quantified. The data are the means ± SE for three independent experiments.
Fig. 5 31P-NMR spectra of intact cells of the wild type and AR3. Log phase cells grown in TAP medium were washed with 10 mM Tris-MES buffer, pH 7.0. The resulting cell suspension, which was concentrated to the density of 5 (mg Chl) ml–1, was transferred to a sample tube. 31P-NMR spectra were measured with a Bruker PDX400 NMR spectrometer, and a 5 mm diameter QNP probe head operating at 162.2 MHz in the pulsed Fourier transform mode without proton decoupling and unlocked at room temperature. A total of 1,024 scans were accumulated for each spectrum with line broadening of 5.0 Hz. Chemical shifts were measured in ppm relative to external 85% Pi (w/w). Identical trends were observed in another experiment.
Fig. 6 Effects of the concentrations of Pi and arsenate on the growth of the wild type and AR3. (a) Growth in medium containing both 1 mM Pi and various concentrations of arsenate. (b) Growth in medium containing both 1 mM arsenate and various concentrations of Pi. Cells were cultured in each medium for 1 week.
Fig. 7 Concentration dependency of arsenate uptake in the wild type (filled circles) and AR3 (open circles). Log phase cells grown in TAP medium were pre-incubated in the Pi-free TAP medium for 20 min, and then arsenate uptake at appropriate concentrations was measured in the absence of Pi for 5 min. The data obtained by two independent experiments are plotted.
Fig. 8 Homology of PTB1 and PTB2 with other Na+/Pi co-transporters. (a) Alignment of a part of the N-terminal domains of Na+/Pi co-transporters. (b) Alignment of a part of the C-terminal domains of Na+/Pi co-transporters. PTB1, C. reinhardtii Pi transporter homolog/accession number AB074880; PTB2, C. reinhardtii Pi transporter homolog/accession number AB074881; PHO89, Saccharomyces cerevisiae probable Na+/Pi symporter, pho89p/accession number P38361; PHO4, Neurospora crassa Pi-repressible Pi permease/accession number P15710; A. thaliana, Arabidopsis thaliana putative Pi transporter/accession number X98130; M. crystallium, Mesembryanthemum crystallinum probale Pi permease/accession number T12576; T. brucei, Trypanosoma brucei possible Pi-repressible Pi permease/accession number AL359782; Nostoc sp. PCC7120, Nostoc sp. PCC7120 Pi permease/Kaneko et al. 2001. Identical amino acids are boxed.
Inhibition of the initial rates of Pi and arsenate uptake by the counter-anion in the wild type and AR3 of C. reinhardtii
| Concentration of counter-anion | Uptake (nmol mg–1 Chl min–1) | ||||
| Wild type | AR3 | ||||
| Pi | Arsenate | Pi | Arsenate | ||
| Without | 12.9 | 5.1 | 42.5 | 9.9 | |
| 1 mM | 8.0 (38%) | 1.8 (65%) | 21.4 (50%) | 3.3 (67%) | |
| 10 mM | 5.5 (57%) | 0.5 (90%) | 5.9 (86%) | 0.4 (96%) | |
| Concentration of counter-anion | Uptake (nmol mg–1 Chl min–1) | ||||
| Wild type | AR3 | ||||
| Pi | Arsenate | Pi | Arsenate | ||
| Without | 12.9 | 5.1 | 42.5 | 9.9 | |
| 1 mM | 8.0 (38%) | 1.8 (65%) | 21.4 (50%) | 3.3 (67%) | |
| 10 mM | 5.5 (57%) | 0.5 (90%) | 5.9 (86%) | 0.4 (96%) | |
The initial rates of Pi uptake at 1 mM in the presence of various concentrations of arsenate, and those of arsenate uptake at 1 mM in the presence of various concentrations of Pi were determined. Numbers in parentheses are percentage inhibition. Identical trends were observed in another experiment.
Inhibition of the initial rates of Pi and arsenate uptake by the counter-anion in the wild type and AR3 of C. reinhardtii
| Concentration of counter-anion | Uptake (nmol mg–1 Chl min–1) | ||||
| Wild type | AR3 | ||||
| Pi | Arsenate | Pi | Arsenate | ||
| Without | 12.9 | 5.1 | 42.5 | 9.9 | |
| 1 mM | 8.0 (38%) | 1.8 (65%) | 21.4 (50%) | 3.3 (67%) | |
| 10 mM | 5.5 (57%) | 0.5 (90%) | 5.9 (86%) | 0.4 (96%) | |
| Concentration of counter-anion | Uptake (nmol mg–1 Chl min–1) | ||||
| Wild type | AR3 | ||||
| Pi | Arsenate | Pi | Arsenate | ||
| Without | 12.9 | 5.1 | 42.5 | 9.9 | |
| 1 mM | 8.0 (38%) | 1.8 (65%) | 21.4 (50%) | 3.3 (67%) | |
| 10 mM | 5.5 (57%) | 0.5 (90%) | 5.9 (86%) | 0.4 (96%) | |
The initial rates of Pi uptake at 1 mM in the presence of various concentrations of arsenate, and those of arsenate uptake at 1 mM in the presence of various concentrations of Pi were determined. Numbers in parentheses are percentage inhibition. Identical trends were observed in another experiment.
Abbreviations
- AR
arsenate-resistant
- AS
arsenate-sensitive
- NMR
nuclear magnetic resonance
- RT–PCR
reverse transcription–polymerase chain reaction
References
Bun-ya, M., Shikata, K., Nakade, S., Yompakdee, C., Harashima, S. and Oshima, Y. (
Cobbett, C. and Goldsbrough, P. (
Fujiwara, S., Kobayashi, I., Hoshino, S., Kaise, T., Shimogawara, K., Usuda, H. and Tsuzuki, M. (
Ha, S.B., Smith, A.P., Howden, R., Dietrich, W.M., Bugg, S., O’Connell, M.J., Goldsbrough, P.B. and Cobbett, C.S. (
Hartley-Whitaker, J., Ainsworth, G., Vooijs, R., Ten Bookum, W., Schat, H. and Meharg, A.A. (
Kaise, T., Hanaoka, K., Tagawa, S., Hirayama, T. and Fukui, S. (
Kaneko, T., Nakamura, Y., Wolk, C.P., Kuritz, T., Sasamoto, S., et al. (
Kobayashi, I., Fujiwara, S., Shimogawara, K., Kaise, T., Usuda, H. and Tsuzuki, M. (
Lee, D.A., Chen, A. and Schroeder, J.I. (
Ma, L.Q., Komar, K.M., Cong, Tu., Zhang, W., Cai, Y. and Kennelley, E.D. (
Maitani, T., Kubota, H., Sato, K. and Yamada, T. (
Meharg, A.A. and Macnair, M.R. (
Pickering, I.J., Prince, R.C., George, M.J., Smith, R.D., George, G.N. and Salt, D.E. (
Rosenberg, H., Gerdes, R.G. and Chegwidden, K. (
Ruiz, J.M. and Romero, L. (
Sharples, J.M., Meharg, A.A., Chambers, S.M. and Cairney, J.W.G. (
Shimogawara, K., Wykoff, D.D., Usuda, H. and Grossman, A.R. (
Schmöger, M.E.V., Oven, M. and Grill, E. (
Willsky, G.R. and Malamy, M.H. (