-
PDF
- Split View
-
Views
-
Cite
Cite
Katharina Nowak, Nora Luniak, Christina Witt, Yvonne Wüstefeld, Andreas Wachter, Ralf R. Mendel, Robert Hänsch, Peroxisomal Localization of Sulfite Oxidase Separates it from Chloroplast-based Sulfur Assimilation, Plant and Cell Physiology, Volume 45, Issue 12, 15 December 2004, Pages 1889–1894, https://doi.org/10.1093/pcp/pch212
Close - Share Icon Share
Abstract
Recently, we isolated the sulfite oxidase (SO) gene from Arabidopsisthaliana and characterized the purified SO protein. The purpose of the present study was to determine the subcellular localization of this novel plant enzyme. Immunogold electron-microscopic analysis showed the gold labels nearly exclusively in the peroxisomes. To verify this finding, green fluorescent protein was fused to full-length plant SO including the putative peroxisomal targeting signal 1 (PTS1) ‘SNL’ and expressed in tobacco leaves. Our results showed a punctate fluorescence pattern resembling that of peroxisomes. Co-labelling with MitoTracker-Red excluded that the observed fluorescence was due to mitochondrial sorting. By investigation of deleted or mutated PTS1, no functional peroxisomal targeting signal 2 (PTS2) could be detected in plant SO. This conclusion is supported by expression studies in Pichia pastoris mutants with defined defects either in PTS1- or PTS2-mediated peroxisomal import.
(Received August 17, 2004; Accepted October 8, 2004)
Sulfite oxidase (SO) from vertebrate sources is one of the best studied molybdenum (Mo) enzymes (EC 1.8.3.1). Eukaryotic SO is a dimeric enzyme and each monomer harbours two prosthetic groups: a haem and a molybdenum cofactor (Moco) domain. SO catalyses the reaction SO32– + H2O → SO42– + 2H+ + 2e–, which is the important terminal step in the oxidative degradation of cysteine, methionine and membrane components such as sulfatides. In mammals, SO is localized in the intermembrane space of mitochondria (Cohen et al. 1972), where electrons derived from sulfite are passed via the haem domain on to cytochrome c, the physiological electron acceptor.
In plants, the existence of SO was a matter of controversy for a long time. During primary sulfate assimilation in the chloroplast, sulfate is reduced via sulfite to organic sulfide, which is essential for cysteine biosynthesis (Leustek and Saito 1999). However, it has also been reported that sulfite can be oxidized back to sulfate, e.g. when plants were subjected to SO2 gas (as reviewed in Heber and Hüve 1998). Sulfite oxidation in intact chloroplasts is enhanced by light and is sensitive to inhibitors of the photosynthetic electron transport (Dittrich et al. 1992, Jolivet et al. 1995). It was interpreted as being be due to non-enzymic reactions during electron transport. Thus, SO activity would counteract sulfate assimilation, provided that this enzyme was localized in the chloroplast.
Recently, work from our laboratory has identified SO as the fourth member of the Mo enzymes in plants (Eilers et al. 2001). Among eukaryotes, plant SO lacks the haem domain and is therefore the smallest Mo enzyme known to date. In silico analysis of plant SO revealed that the protein contains a C-terminal SNL tripeptide. This shows high homology to the C-terminal amino acid sequence serine-lysine-leucine (SKL) which is the consensus peroxisomal targeting signal 1 (PTS1) and is sufficient to direct polypeptides to peroxisomes in vivo in plants, animals and yeast (Hayashi et al. 1996, Mullen et al. 1997a). Plant PTS1 motifs apparently exhibit more variability in sequence compared with accepted signals in animals (Mullen et al. 1997a). Furthermore, the efficiency of protein sorting by a PTS1 can be enhanced by adjacent residues (Mullen et al. 1997a, Mullen et al. 1997b). Additionally a PTS2, located near the N-terminus of plant SO, was postulated. The PTS2 has a loosely conserved sequence of nine amino acids (Flynn et al. 1998, Olsen 1998). In plants and mammals, the PTS2 is cleaved after translocation.
In our studies we investigated the subcellular localization of plant SO by using (i) immunogold electron microscopy, and (ii) transient expression of SO fused to the reporter green fluorescent protein (GFP) gene. Our detailed analysis using different methods was necessary because of contradictory reports in the literature: animal SO was found in mitochondria (Cohen et al. 1972), and previous cell fractionation studies showed plant SO activity only in the chloroplast fraction (Dittrich et al. 1992, Jolivet et al. 1995). Now that the gene for Arabidopsis SO has been cloned (Eilers et al. 2001) and antiserum against plant SO is available, this question can be ultimately answered. First, preliminary data using cell fractionation followed by immunodetection of SO showed a strong signal in the mitochondrial/peroxisomal fraction and a weaker one in peroxisomes (Eilers et al. 2001). The data presented here using two different techniques independently and unequivocally confirm that the plant SO studied (accession no. AF200972) is localized exclusively in peroxisomes and not in mitochondria, chloroplasts or the cytoplasm. Hence SO activity does not interfere with the chloroplast-localized sulfur assimilation pathway. Rather, it might be speculated that peroxisomal-localized plant SO has a sulfite-detoxifying function as shown for the animal enzyme in mitochondria. Possible explanations for the ‘new’ localization of the plant enzyme come from (i) the need for a new electron acceptor other than cytochrome c because plant SO lacks the haem domain present in the animal enzyme, and (ii) a possible role for protecting catalase. Veljovic-Javanovic et al. (1998) have shown that peroxisomal catalase is inhibited by low amounts of sulfite—here plant SO could play an important role in protecting this enzyme.
Subcellular localization of plant SO using immunogold
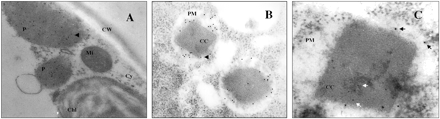
Immunogold experiments were performed on ultrathin sections of Arabidopsis thaliana leaves and of protoplast-derived microcolonies of Nicotiana plumbaginifolia using affinity-purified anti-SO antibody (Yamaya et al. 1992) against A. thaliana SO, followed by a goat anti-rabbit antibody conjugated with 10 nm gold. Fig. 1 shows representative transmission electron micrographs illustrating the subcellular localization of plant SO in A. thaliana and N. plumbaginifolia, respectively.
Immunolabelling micrographs were quantified by counting the numbers of gold particles over the whole cell. Only low background labelling was detected in other organelles like mitochondria or chloroplasts, and no gold particles were seen in cytoplasm, cell wall or nucleus (Table 1). Furthermore, in control experiments performed with the appropriate pre-immune serum of the SO antibody no non-specific labelling of peroxisomes and other organelles or cell wall was seen (data not shown). The matrix of peroxisomes was labelled at a density of 144 and 65 gold particles per µm2 for Arabidopsis and N. plumbaginifolia, respectively while the catalase crystal showed only a quarter of this amount. The density of labelling was in the range of other peroxisomal proteins as previously shown for malate synthase in Arabidopsis (Olsen et al. 1993) and for isocitrate lyase in castor bean (Gao et al. 1996).
Because of the nearly identical size of peroxisomes and mitochondria a morphological differentiation between the two organelles is important when interpreting micrographs. In Fig. 1B one can detect catalase crystals that occur only in peroxisomes but not in mitochondria. Also double labelling (Geuze et al. 1981) with anti-urease antibody (Sigma, Germany) proved that SO is only present in peroxisomes. Co-incubation with two specific antisera, one directed against A. thaliana SO and the other against the peroxisome marker urease, and final labelling with secondary antibodies conjugated with 10 nm (for SO) and 5 nm (for urease) gold particles (Fig. 1C), demonstrated co-localization of SO and urease within the same organelle.
Subcellular localization of plant SO using GFP in plant cells
To confirm the results of the immunogold experiments we used GFP as a reporter gene to monitor subcellular localization of SO protein. Experiments were performed with Nicotiana tabacum L. cv. Gatersleben (GAT). Plants were grown in soil culture in controlled environment chambers (HPS 1500; Hereaus-Vötsch, Balingen, Germany) with 14 h daily light (2,000 lx) periods and relative humidity of 80%. Young leaves of tobacco plants were harvested and placed upside down on water-soaked filter paper in Petri dishes, and transformed with the Particle Delivery System PDS-1000 (Bio-Rad, Munich, Germany) as described earlier (Nowak et al. 2004). In co-transformation experiments, both DNAs were precipitated on to the gold particles with half of the amount compared with the standard protocol.
GFP/yellow fluorescent protein (YFP) expression was visualized with the confocal laser scanning microscope CLSM-510META (Carl Zeiss, Göttingen, Germany). An argon laser line of 488 nm was used to excite the GFP and YFP in fluorochromes and chlorophyll, respectively. Emission spectra of the excited GFP and the chlorophyll autofluorescence were collected in two separate channels, 505–550 nm and 665–719 nm, respectively. For double-labelling experiments (YFP and GFP), the images were recorded with the lambda mode of the META channel, and subsequent spectral separation of fluorescence signals was performed with the unmixing software of the CLSM510 META (for more details see Nowak et al. 2004). MitoTracker-Red (CMXRos; Molecular Probes, The Netherlands) was used to stain mitochondria. Stripped lower epidermis of leaves was incubated for 30 min in a 1 µM dye solution containing 50 mM HEPES (pH 7.0) and 330 mM sorbitol, and washed three times in dye-free buffer to eliminate excess dye. A HeNe laser line of 543 nm was used to excite MitoTracker-Red. Images were recorded using the multitracking mode and emitted light (at 569–623 nm) was detected with the META channel. If necessary, the bright field of samples was taken using the transmitted light photomultiplier.
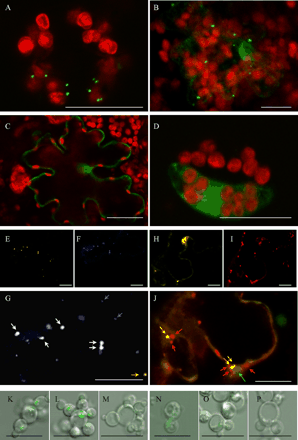
The fusion protein GFP::SO-SNL consisted of full-length Arabidopsis SO (including the C-terminal SNL of SO and immediately followed by the stop codon) fused to the C-terminus of GFP. In confocal laser scanning microscopy (CLSM) analyses a punctate fluorescence pattern was observed that was interpreted as resembling peroxisomes (Fig. 2A). The overlay of chlorophyll autofluorescence demonstrates that GFP was excluded from the chloroplasts. To distinguish between peroxisomes and mitochondria and to prove that the observed fluorescence is not a result of mitochondrial targeting, we performed double transformation experiments with different fluorescent proteins, and stained mitochondria with MitoTracker-Red. Tobacco leaves were co-transformed with GFP::SO-SNL and with the control construct pFF19-YFP-SKL for peroxisome targeting. The latter control construct (Mathur et al. 2002) comprises YFP and the motif SKL at its C-terminus. SKL is the proposed consensus motif of the PTS1, which is responsible for import of peroxisome-destined proteins. If SO is actually targeted to peroxisomes co-localization of both fusion proteins should be visible. The separated fluorescence images and the overlay are shown in Fig. 2E–G. In the merged image (Fig. 2G), most of the GFP dots overlay exactly those of YFP giving white spots as the merged colour, thus indicating that GFP::SO-SNL is targeted to peroxisomes like the control pFF19-YFP-SKL. The reason for a less than 100% co-localization of the two proteins might be that (i) co-localizations are always statistical processes, and (ii) especially from peroxisomes it is known that protein filling takes place only in a limited time frame; fully developed peroxisomes stop their import of proteins. Therefore it is possible to have either peroxisomes with the two proteins in nearly the same amount or to find peroxisomes with a lower amount of only one protein and therefore a hardly detectable fluorescence of one colour. Additionally, staining of mitochondria with MitoTracker-Red was performed in samples transformed with GFP::SO-SNL (Fig. 2H–J). It can be seen that (i) two organelles of nearly identical size but with different colours are present, and that (ii) there is no overlapping between the GFP and the red colour of MitoTracker.
In order to analyse the efficiency of peroxisomal import by the PTS1 motif, the tripeptide SNL at the C-terminus of GFP::SO was deleted or replaced by the tripeptides SGL and ANG. Fig. 2A–D includes representative images illustrating the subcellular localization of these GFP::SO fusion proteins in transformed tobacco cells. Fig. 2B shows a punctate fluorescence pattern attributable to the transient expression of GFP::SO-SGL where asparagine of the SNL motif was replaced by the smaller amino acid glycine. Only in a few cases was additional fluorescence detected in the cytosol and nucleus of the transformed cells. This implies that SGL is sufficient to direct the majority of GFP::SO-SGL molecules from the cytosol to peroxisomes. This was not expected since SGL does not conform strictly to the proposed consensus motifs of plant PTS1 (A/C/G/S/T–H/K/L/N/R–I/L/M/Y) (Mullen et al. 1997a). Furthermore Mullen (2002) reported that G at the –2 position is not functional. Hence it follows that the proper functioning of PTS1 is context dependent in plant SO. This phenomenon was noticed earlier and it was suggested (Elgersma et al. 1996, Mullen et al. 1997a) that in cases where PTS1 does not conform to the SKL consensus motif, residues upstream of the PTS1 convey the proper context for the tripeptide signal to function. Probably the best example for the context role of adjacent residues in PTS1 targeting comes from studies of cotton seed catalase (Mullen et al. 1997b). The C-terminal tripeptide -PSI is conspicuously divergent from the SKL motif. Targeting studies revealed that -PSI is necessary, but interestingly not sufficient, for sorting to peroxisomes. However, when an R (a residue commonly found at the –4 position in plant catalases) was added to the C-terminal -PSI, the tetrapeptide was sufficient for sorting chloramphenicol acetyltransferase fusion proteins to peroxisomes. In fact, a basic residue directly precedes the PTS1 of Arabidopsis SO (-HSNL). Since other plant SO sequences also possess this basic residue, e.g. rice (-RSKM), poplar (-HSNM) and rapeseed (-HSNL), we suggest the importance of a basic residue at the –4 position for peroxisomal targeting.
However, fluorescence of GFP::SO-ANG was restricted to the cytosol and nucleus of transformed tobacco cells (Fig. 2C). This indicates that within the SNL motif the substitution of serine with alanine and of leucine with glycine is not tolerated for proper sorting of GFP::SO-ANG into peroxisomes. In this case, even the basic histidine at the –4 position is obviously not able to compensate for the changed residues within the tripeptide of plant SO. This provides additional evidence for SKL as the plant PTS1 consensus motif (Mullen et al. 1997a).
To test the requirement of SNL for peroxisomal import and to elucidate whether another PTS, namely a cryptic PTS2, is involved in targeting the protein we transiently expressed GFP::SO-DEL and SO-DEL::GFP harbouring no PTS1 at the C-terminus. As shown in Fig. 2D for the N-fusion construct (SO-DEL::GFP), peroxisomal targeting was lost and fluorescence accumulated only throughout the cytosol and the nucleus.
Therefore we can conclude that (i) import of plant SO into peroxisomes is dependent only upon the presence of its PTS1 motif—which is necessary and sufficient for proper sorting of the protein to the organelle, and that (ii) other functional signals like a cryptic PTS2 are not present within Arabidopsis SO.
Subcellular localization of plant SO using GFP in Pichia pastoris pex mutants
Localization experiments were also performed in Pichia pastoris.All strains were kindly provided by S. Subramani (San Diego, CA, U.S.A.). Knowing the shortfalls of transient expressions—artificially expressed proteins in large amounts sometimes cause mislocalization in yeast cells—only data obtained from stably transformed Pichia cells are shown in this paper. The GFP fusion constructs GFP::SO-SNL, GFP::SO-DEL and GFP–SKL were cloned into the Pichia vector pPICZA and were stably transformed by electroporation according to the EasySelect™ Pichia Expression Kit Protein Expression user’s manual (Invitrogen, The Netherlands) into the Pichia wild-type strain PPY12 and also into the peroxisomal import mutants ΔPex5p and ΔPex7p, which are defective in PTS1-mediated import and PTS2-mediated import, respectively (Elgersma et al. 1998). Fig. 2K–P include representative images illustrating the subcellular localization of the respective GFP fusion proteins. The control construct GFP–SKL transformed in the wild-type strain PPY12 was localized in peroxisomes and other cellular structures were not labelled (Fig. 2K). Microscopic analysis of GFP–SKL transformed into ΔPex7p, a mutant defective in import via PTS2, showed the same localization as observed for the wild-type strain (Fig. 2L), i.e. accumulation in peroxisomes. Targeting of GFP–SKL into peroxisomes was abolished when it was expressed in ΔPex5p where the fluorescence remained in the cytosol (Fig. 2M). These experiments were repeated with the SO fusion protein. GFP::SO-SNL transformed into PPY12 (Fig. 2N) and ΔPex7p (Fig. 2O), respectively, revealed a peroxisomal localization similar to the control construct GFP–SKL. However, the fluorescence signals detected were much weaker than those obtained for the control GFP–SKL because of the toxicity of the overexpressed SO protein for Pichia. We also expressed GFP::SO-SNL in ΔPEX5p (Fig. 2P), which lacks a functional PTS1 receptor. Microscopic analysis showed that the weak fluorescence observed was distributed throughout the cytosol and no accumulation of GFP could be detected in peroxisomes. These results indicate that the tripeptide SNL is sufficient and necessary for targeting of plant SO into peroxisomes of Pichia.
Furthermore, we expressed GFP::SO-DEL harbouring no PTS1 at the C-terminus in P. pastoris strains to elucidate whether other peroxisomal targeting signals are involved in protein targeting. In all Pichia strains transformed with this fusion construct, fluorescence was only distributed throughout the cytosol, i.e. the chimeric protein was no longer transported into peroxisomes (data not shown). These results demonstrate that besides the PTS1 motif SNL no additional peroxisomal targeting signal is present and responsible for import of the fusion protein into peroxisomes. This is also in agreement with our localization results in tobacco transformed with GFP::SO-DEL.
Plasmid construction
All restriction endonuclease and ligase reactions were performed using the buffer conditions recommended by their respective manufacturers using standard techniques (Sambrook et al. 1989). The pFF19-GFP-SKL has been described previously (A. Wachter, S. Wolf, H. Steininger, J. Bogs, T. Rausch, submitted).
The various SO fragments were generated via PCR mutagenesis using Pwo DNA polymerase (Peqlab, Erlangen, Germany) with SOpQE60 (Eilers et al. 2001) as template. The N-fusion construct was generated via PCR using the primer set: N-SO-DELSNLfor 5′-TCC ATG GCT GGA ATT AGA GGT CCT TCG GAA-3′ and N-SO-DELSNLrev 5′-AGA TCT GTG GCC AAG CCG GAG AAG GAC ACG GTG-3′.
For the C-fusion constructs the PCR mixture included a forward primer (C-SO-NcoI: 5′-CCA TGG CTG GAA TTA GAG GTC CTT CGG-3′) and an appropriate reverse primer, depending on the desired modification. The following list shows the reverse primers used, which introduced a BglII site and replaced or deleted the SNL motive of plant SO (exception: SO with unaltered PTS1): C-SO-SGL-STOPrev: 5′-AGA TCT CTA CAA GCC AGA GTG GCC AAG CCG GAG AGG ACA CGG-3′; C-SO-ANG-STOPrev: 5′-AGA TCT CTA CCC GTT AGC GTG GCC AAG CCG GAG AAG GAC ACG G-3′; C-SO-DELSNL-STOPrev: 5′-AGA TCT CTA GTG GCC AAG CCG GAG AAG GAC ACG G-3′. The PCR products were cloned into pCR®-BluntII-TOPO® (Invitrogen, The Netherlands) and sequenced.
For plant transformation: after NcoI and BglII cutting, the resulting fragments were transferred into pBSK-GFP-C-Fus and pBSK-GFP-N-Fus vectors, respectively. These vectors harbour the CaMV-35S promoter with a double enhancer, the poly(A) tail from cauliflower mosaic virus 35S promoter (CaMV-35S) and GFP provided with NcoI and BglII for N- or C-terminal fusion of SO. pFF19-YFP-SNL used in double-labelling experiments, was created as described previously (Nowak et al. 2004).
For P. pastoris: We used the plant vectors as template for PCR-cloning. The PCR mixture includes a forward primer (5′-GTG CTA GGT ACC ATG GTG AGC AAG GGC GAG GAG CTG-3′) with a KpnI-site and a set of reverse primers with SacII sites: revSO-DEL: 5′-GAT TAC CGT CCG CGG CTA GTG GCC AAG CCG GAG AAG GAC-3′; revSO-SNL: 5′-GAT AAC GTG CGC GGC TAC AAG TTA GAG TGG CCA AAC CG-3′; revGFP-SKL: 5′-GAT ACC GTC CGC GGT TAC AAT TTA GAC TTG TAC AGC TC-3′.
The amplificated GFP fragments were subcloned in pGEM®-T Easy (Promega, Germany) or directly transferred into the pPICZA vector, controlled by the AOX1 promoter.
Acknowledgments
We are grateful to Vijendra K. Sharma for critical comments on the manuscript. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Me 1266/14–2).
Corresponding author: E-mail, r.mendel@tu-bs.de; Fax, +49-531-391-8128.
Fig. 1 Micrographs of immunogold electron microscopy for SO in A. thaliana leaves (A) and N. plumbaginifolia microcolonies (B, C). Secondary antibody for SO was labelled with 10 nm gold particles (black arrow), and additionally in (C) with 5 nm gold for the urease (white arrow). The pictures were taken at a magnification of 20,000× (A), 42,000× (B) and 70,000× (C). Abbreviations: CC, catalase crystal; Chl, chloroplasts; CW, cell wall; Cy, cytoplasm; Mi, mitochondria; P, peroxisomes; PM, peroxisome matrix,
Fig. 2 Expression of fluorescent proteins in tobacco cells (A–J) and P. pastoris (K–P). Transient expression of different GFP–SO constructs are shown: GFP::SO-SNL (A), GFP::SO-SGL (B), GFP::SO-ANG (C) and SO-DEL::GFP (D). Co-localization of YFP-SKL (E) and GFP::SO-SNL (F) is shown in (G). For better clarity in (F), we coloured GFP blue so that white dots as merged colour represent perfect matches of the two fluorescent proteins. A merged image of transient expression of GFP::SO-SNL (H) and labelling with MitoTracker-Red (I) is shown in (J), chlorophyll autofluorescence of chloroplasts is coloured green. Images of P. pastoris strains (wild type: K,N; ΔPex7: L,O; ΔPex5: M,P), stably transformed with GFP–SKL (K–M), GFP::SO-SNL (N–P) are shown. The figures are merged images of DIC (Nomarsky) and GFP fluorescence. For more details see results. The bars represent 20 µm (A–J) and 10 µm (K–P), respectively. Arrows: in (G) blue for GFP::SO-SNL, yellow for YFP-SKL and white for co-localization and in (J) red for MitoTracker, yellow for GFP::SO-SNL and green for a chloroplast.
Gold labelling intensities for SO in organelles of A. thaliana leaves and N.plumbaginifolia protoplast-derived microcolonies, as counted on immuno-electron micrographs
| Localization | Gold particles µm–2 (± SD) a | |
| A. thaliana | N. plumbaginifolia | |
| Peroxisomes | 144 (± 60) | 65 (± 46) |
| Catalase crystal | – | 16 (± 11) |
| Mitochondria | 2 (± 5) | 0 (± 0) |
| Plastids | 3 (± 4) | – |
| Cytoplasm | 1 (± 1) | 0 (± 0) |
| Cell wall | 0 (± 0) | 0 (± 0) |
| Localization | Gold particles µm–2 (± SD) a | |
| A. thaliana | N. plumbaginifolia | |
| Peroxisomes | 144 (± 60) | 65 (± 46) |
| Catalase crystal | – | 16 (± 11) |
| Mitochondria | 2 (± 5) | 0 (± 0) |
| Plastids | 3 (± 4) | – |
| Cytoplasm | 1 (± 1) | 0 (± 0) |
| Cell wall | 0 (± 0) | 0 (± 0) |
a Forty-five micrographs were counted for each species.
Gold labelling intensities for SO in organelles of A. thaliana leaves and N.plumbaginifolia protoplast-derived microcolonies, as counted on immuno-electron micrographs
| Localization | Gold particles µm–2 (± SD) a | |
| A. thaliana | N. plumbaginifolia | |
| Peroxisomes | 144 (± 60) | 65 (± 46) |
| Catalase crystal | – | 16 (± 11) |
| Mitochondria | 2 (± 5) | 0 (± 0) |
| Plastids | 3 (± 4) | – |
| Cytoplasm | 1 (± 1) | 0 (± 0) |
| Cell wall | 0 (± 0) | 0 (± 0) |
| Localization | Gold particles µm–2 (± SD) a | |
| A. thaliana | N. plumbaginifolia | |
| Peroxisomes | 144 (± 60) | 65 (± 46) |
| Catalase crystal | – | 16 (± 11) |
| Mitochondria | 2 (± 5) | 0 (± 0) |
| Plastids | 3 (± 4) | – |
| Cytoplasm | 1 (± 1) | 0 (± 0) |
| Cell wall | 0 (± 0) | 0 (± 0) |
a Forty-five micrographs were counted for each species.
Abbreviations
- CaMV
cauliflower mosaic virus
- CLSM
confocal laser scanning microscopy
- GFP
green fluorescent protein
Moco
molybdenum cofactor
- PTS
peroxisomal targeting signal
- SO
sulfite oxidase
- YFP
yellow fluorescent protein.
References
Cohen, H.J., Betcher-Lange, S., Kessler, D.L. and Rajagopalan, K.V. (
Dittrich, A.P.M., Pfanz, H. and Heber, U. (
Eilers, T., Schwarz, G., Brinkmann, H., Witt, C., Richter, T., Nieder, J., Koch, B., Hille, R., Hänsch, R. and Mendel, R.R. (
Elgersma, Y., Vos, A., van den Berg, M., van Roermund, C.W.T., van der Sluijs, P., Distl, B. and Tabak, H.F. (
Elgersma, Y., Elgersma-Hooisma, M., Wenzel, T., McCaffery, J.M., Farquhar, M.G. and Subramani, S. (
Flynn, C.R., Mullen, R.T. and Trelease, R.N. (
Gao, X., Marrison, J.L., Pool, M.R., Leech, R.M. and Baker, A. (
Geuze, H.J., Slot, J.W., van der Ley, P.A. and Scheffer, R.C.T. (
Hayashi, M., Aoki, M., Kato, A., Kondo, M. and Nishimura, M. (
Heber, U. and Hüve, K. (
Jolivet, P., Bergeron, E., Zimierski, A. and Meunier, J.C. (
Leustek, T. and Saito, K. (
Mathur, J., Mathur, N. and Hülskamp, M. (
Mullen, R.T. (
Mullen, R.T., Lee, M.S., Flynn, R. and Trelease, R.N. (
Mullen, R.T., Lee, M.S. and Trelease, R.N. (
Nowak, K., Luniak, N., Meyer, S., Schulze, J., Mendel, R.R. and Hänsch, R. (
Olsen, L.J., Ettinger, W.F., Damsz, B., Matsudaira, K., Webb, M.A. and Harada, J.J. (
Sambrook, J., Fritsch, E.F. and Maniatis, T. (
Veljovic-Javanovic, S., Oniki, T. and Takahama, U. (