-
PDF
- Split View
-
Views
-
Cite
Cite
Josette-Renée Landry, Arefeh Rouhi, Patrik Medstrand, Dixie L. Mager, The Opitz Syndrome Gene Mid1 Is Transcribed from a Human Endogenous Retroviral Promoter, Molecular Biology and Evolution, Volume 19, Issue 11, November 2002, Pages 1934–1942, https://doi.org/10.1093/oxfordjournals.molbev.a004017
Close - Share Icon Share
Abstract
Human endogenous retroviruses (HERVs) and other long terminal repeat (LTR)–containing elements comprise a significant portion (8%) of the human genome and are likely vestiges of retroviral infections during primate evolution. Many of the HERVs present in human DNA have retained functional promoter, enhancer, and polyadenylation signals, and these regulatory sequences have the potential to modify the expression of nearby genes. To identify retroviral elements that contribute to the transcription of human genes, we screened sequence databases for chimeric (viral-cellular) transcripts. These searches revealed a fusion transcript containing the LTR of an HERV-E element linked to the Opitz syndrome gene Mid1. We confirmed the authenticity of the chimeric transcript by 5′ rapid amplification of cDNA ends (RACE) and established that the Mid1 mRNA isoform was transcribed from a retroviral LTR. The identification of a retroviral first exon suggested the existence of alternative promoters for Mid1 because nonretroviral (native) 5′ untranslated regions (UTRs) had been reported previously for this gene. Although Mid1 transcripts could be detected in all tissues tested, quantitative real-time reverse transcription–polymerase chain reaction indicated that the retroviral promoter contributes significantly to the level of Mid1 transcripts in placenta and embryonic kidney, where chimeric mRNAs were found to represent 25% and 22% of overall Mid1 mRNAs, respectively. Transient transfection studies supported a role for the LTR as a strong tissue-specific promoter in placental and embryonic kidney cell lines and suggested a function for the LTR as an enhancer. These findings provide further evidence that some endogenous retroviruses have evolved a biological function by contributing transcriptional regulatory elements to cellular genes.
Introduction
The human genome harbors several classes of retroelement sequences which together account for over 40% of nuclear DNA (International Human Genome Sequencing Consortium 2001 ). Retroelements are present in most, if not all, eukaryotes, where they proliferated during evolution through the reverse transcription of RNA intermediates followed by reintegration of the new copies into the host's genome. In humans, short and long interspersed elements (reviewed in Mighell, Markham, and Robinson [1997] ; Kazazian and Moran [1998] ), represented primarily by Alu and L1 repeats, make up the majority of this group of repetitive sequences. Human endogenous retroviruses (HERVs) (Wilkinson, Mager, and Leong 1994 ; Lower R, Lower J, and Kurth 1996 ) and other long terminal repeat (LTR)–like elements follow in prevalence and account for an estimated 8% of the human genome (International Human Genome Sequencing Consortium 2001 ).
The thousands of HERVs and solitary LTRs in primate DNA are believed to be the remnants of ancient retroviral germ-line infections because intact elements closely resemble exogenous retroviruses in genomic organization. Although the vast majority of HERVs are now unable to code for retroviral proteins, many have remained transcriptionally active and still possess active regulatory elements within their LTRs (Sverdlov 2000; Baust et al. 2001 ). In addition to their natural role in the transcription of retroviral genes, the promoter and enhancer sequences of some HERVs have acquired novel cellular functions by participating in the expression of neighboring genes (Ting et al. 1992 ; Di Cristofano et al. 1995 ; Schulte et al. 1996 ; Bi et al. 1997 ; Medstrand, Landry, and Mager 2001 ). HERV-E elements, more specifically, have been shown to participate in the regulation of several human genes by donating a tissue-specific enhancer (Samuelson et al. 1990 ; Ting et al. 1992 ) to the salivary amylase gene and by contributing alternative promoters to the apolipoprotein-C1 and endothelin-B receptor genes (Medstrand, Landry, and Mager 2001 ). Through bioinformatic analysis, we have determined that an HERV-E element is also involved in transcriptional regulation of the human Mid1 gene.
Mutations in Mid1 have been recently implicated in the pathogenesis of the X-linked form of Opitz syndrome (Quaderi et al. 1997 ; Gaudenz et al. 1998 ), a genetic disorder that primarily affects the development of midline structures (Opitz 1987 ; Robin, Opitz, and Muenke 1996 ). Mid1 is a single-copy gene that encodes a microtubule-associated protein (Cainarca et al. 1999 ; Schweiger et al. 1999 ) which has been reported to associate with (Liu et al. 2001 ) and target phosphatase 2A for degradation (Trockenbacher et al. 2001 ). Whereas several studies have focused on the function of the Mid1 gene product, transcriptional regulation of the Mid1 gene has received relatively little attention. Although multiple Mid1 transcript sizes as well as several alternative 5′ untranslated regions (UTRs) have been described (Quaderi et al. 1997 ; Perry et al. 1998 ; Van den Veyver et al. 1998 ; Cox et al. 2000 ), the promoter or promoters of Mid1 have remained uncharacterized. Here, we report that a retroviral element acts as an alternative tissue-specific promoter for this gene.
Materials and Methods
Database Searches and Sequence Analysis
The human expressed sequence tag (EST) and nonredundant (nr) databases were screened by BLAST version 2.0 (Altschul et al. 1997 ) using HERV-E leader regions as query sequences to identify novel chimeric mRNAs. Transcripts identified were analyzed by Repeatmasker with RepBase version 3.04 (http://ftp.genome.washington.edu/cgi-bin/RepeatMasker) to assess whether they contained nonretroviral sequence, and potential hybrids were further characterized to determine the nature of the nonrepetitive sequence by conducting further BLAST searches. The analysis for transcription factor binding sites was performed using Alibaba2.1 (http://www.iti.cs.uni-magdeburg.de/∼grabe/alibaba2/).
Rapid Amplification of cDNA Ends
All oligonucleotides used for rapid amplification of cDNA ends (RACE) and other procedures are listed in table 1 . 5′ RACE was performed using a Marathon-ready placenta cDNA library (Clontech) according to the manufacturer's protocol. The first round of polymerase chain reaction (PCR) amplification was carried out using oligos 45 and AP1 (the latter, provided by the supplier). The nested round of amplification was performed using oligos 46 and AP2. The resulting RACE products were cloned into the pGEM-T vector (Promega) and were hybridized using oligo 47 as a probe. Positive clones were sequenced using ABI Prism Big Dye Cycle Sequence Ready Reaction Kit (PE Applied Biosystems).
Reverse Transcription and Real-Time PCR
Total RNA from human adult and fetal tissues was purchased from Clontech and Stratagene or was prepared from different sections of human placenta as described previously (Wilkinson et al. 1990 ). After the elimination of remaining genomic DNA with DNAse (Gibco-BRL), first-strand cDNA was synthesized as described previously (Medstrand, Lindeskog, and Blomberg 1992 ) using random primers, SuperScript II Reverse Transcriptase (Gibco-BRL), and 10 μg RNA.
Real-time PCR was performed on one-hundredth volume of cDNA using 25 μl of 2 × Sybr green PCR master mix (PE Applied Biosystems) and under the following amplification conditions: 30 s at 95°C, 30 s at 55°C, and 30 s at 72°C for 35 cycles on a Biorad iCycler. Primers 48 and 49 (located in exons 2 and 3, respectively) were used to amplify all Mid1 transcripts; primers 36 and 47 (located in the retroviral first exon, 1R, and exon 2) were used to amplify only Mid1 transcripts containing HERV-E sequence; and primers 60 and 61 were used to amplify all glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts. All primer pairs were designed in accordance with the PE Applied Biosystems guidelines to ensure that the amplification efficiencies of the different primer pairs were almost equal. For that purpose, all primer pairs amplified sequences of similar length (100 bp). In addition, validation experiments were conducted to confirm that the amplification efficiency of the targets (Mid1 retroviral exon 1–exon 2 fragment and exon 2–exon 3 fragment) and reference (GAPDH) products were very similar. Dissociation curves were run to detect nonspecific amplification, and it was determined that single products were amplified in each reaction. The relative quantitation of Mid1 expression was calculated using the comparative threshold cycle method (PE Applied Biosystems User Bulletin #2, ABI PRISM 7700 Sequence Detection System). Levels of Mid1 amplification (all 5′ UTR isoforms) were normalized by GAPDH and expressed relative to the level of Mid1 transcripts in fetal spleen, which was given an arbitrary value of 1 because it contained the least amount of Mid1 mRNA in the various tissues tested.
Plasmid Constructions
The retroviral promoter construct was designed by cloning the 5′ LTR of the Mid1 HERV-E element into the KpnI-BglII site of the pGL3 basic luciferase vector (Promega). The 488-bp LTR was amplified from genomic DNA using primers 11 and 12 followed by a nested PCR using primers 84 and 10. For the native promoter construct, a 1.1-kb fragment upstream of the nonretroviral Mid1 first exon (accession number, Y13667) was amplified using primers 91 and 92 and was inserted in the KpnI-BglII site of pGL3b. The promoter region represented positions −949 to +184 relative to the transcription start site of the nonretroviral Mid1 5′ UTR. Mid1 LTR enhancer plasmids were constructed by amplifying the LTR from human genomic DNA using flanking oligos 11 and 12 and nested primers 101 and 102, then inserting the HERV-E LTR in either orientation in the BamHI site of the promoter construct.
Progressive 5′ deletion constructs of the retroviral promoter were generated by amplifying the LTR with the following primers: 85 and 10 for the fragment from positions 263 to 488 of the LTR, oligos 98 and 10 for positions 179–488, oligos 106 and 10 for positions 150–488, oligos 105 and 10 for positions 119–488, oligos 99 and 10 for positions 96–488, and oligos 113 and 10 for positions 51–488. The resulting LTR sections were then cloned in the KpnI-BglII site of pGL3b.
The marmoset Mid1 native promoter plasmid was constructed by amplifying the region orthologous to the 1.1-kb human Mid1 native promoter from marmoset cell line genomic DNA using oligos 91 and 92 and cloning the resulting product in the KpnI-BglII site of pGL3b. The LTR marmoset promoter construct was generated as described above for the LTR human promoter construct using human genomic DNA for the amplification of the LTR because the HERV-E element is not present in the marmoset Mid1 locus. All constructs were sequenced to confirm orientation and sequence integrity.
Cell Culture and Transient Transfections
HepG2 and 293 cells were cultured in Dulbecco's minimal essential media supplemented with 10% fetal calf serum and antibiotics. Jeg-3 cells were maintained in RPMI supplemented with 5% fetal calf serum and antibiotics. HepG2 cells were seeded 24 h before transfection in six-well plates at a density of 3 × 105 cells per well. Monolayers were cotransfected with 1.5 μg of plasmid DNA and 50 ng of the luciferase vector pRL-TK using calcium phosphate (Cellphect) as described by the supplier. Jeg-3 and 293 cells were seeded in six-well plates at a density of 2 × 105 cells per well and were cotransfected 24 h later with 1.0 μg of plasmid DNA and 200 ng of pRL-TK using 7 μl of Lipofectamine (Gibco-BRL). Transfected cells were washed 24 h later in phosphate-buffered saline and were harvested in 500 μl of 1 × passive lysis buffer (Promega). Firefly and luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega). The data were normalized to the internal Renilla luciferase control and were expressed as the relative expression with respect to pGL3p (SV40 promoter).
Genomic Typing for the Mid1 HERV-E LTR
Genomic DNA was prepared from marmoset (New World Monkey), baboon, gibbon, orangutan, gorilla, chimpanzee, and human cell lines as described previously (Goodchild, Wilkinson, and Mager 1993 ). Primers 12 (situated upstream of the HERV-E element) and 70 (situated in the 5′ LTR) were used in the PCR amplification to detect the presence or absence of the HERV-E element in the Mid1 locus of different primates. After amplification, the PCR products were hybridized to the human 5′ Mid1 HERV-E LTR to confirm the identity of the amplicons.
Results
Identification and Characterization of the Mid1 Chimeric Transcript
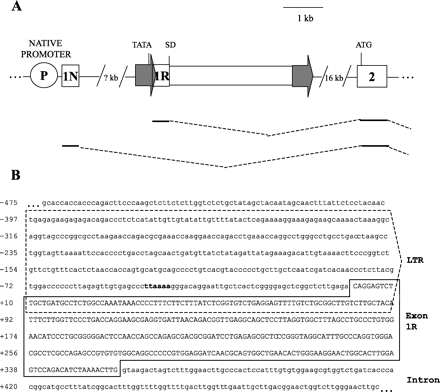
The present study was initiated to identify endogenous retroviruses that participate in the regulation of human genes by providing promoter elements. Because transcripts derived from LTR promoters contain retroviral sequence at their 5′ termini, we searched the human EST and nr databases for hybrid (viral-cellular) mRNAs. These searches revealed a chimeric transcript under accession number AF041208 that had the HERV-E LTR-leader sequence at its 5′ end but continued into the sequence of the second exon of the Mid1 gene. It should be noted that this chimeric transcript was isolated from a fetal kidney cDNA library by Van den Veyver et al. (1998) who reported the presence of an LTR in the 5′ UTR of one of their clones but did not characterize the origin or structure of the hybrid mRNA. Database analysis of the Mid1 genomic region indicated that the HERV-E element involved in the creation of the hybrid mRNA is approximately 4 kb in length and resides 16 kb upstream of the second exon of Mid1 (see fig. 1A ). Generation of the chimeric transcript resulted from a splicing event between the natural splice donor site in the HERV-E element and the splice acceptor site at the beginning of the second exon of Mid1. As a result, the chimeric mRNA generated was nearly identical to a previously reported (Quaderi et al. 1997 ) nonchimeric Mid1 transcript (in accession Y13667) with the exception of the first exon. Because the translation initiation site (ATG) of Mid1 occurs in exon 2, the chimeric transcript likely encodes a normal Mid1 protein while possessing an alternative 5′ UTR.
We confirmed the existence and transcriptional initiation site of this chimeric transcript by performing 5′ RACE on placental cDNA. Sequence analysis of 19 RACE clones led to the identification of 7 hybrid clones as well as 12 nonretroviral clones containing heterogenous sequences, supporting the existence of alternative first exons for Mid1. Moreover, the three longest of the seven hybrid clones all started 34 nucleotides further 5′ than the original EST clone AF014108. As shown in figure 1B, the three longest 5′ RACE clones initiated 40 bp downstream of the putative retroviral TATA box in the LTR. These results strongly suggest that the Mid1 mRNA isoform is transcribed from a retroviral promoter.
Tissue Specificity of the Chimeric Mid1 Transcript
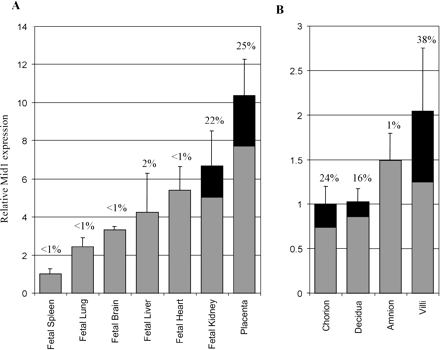
The 5′ RACE results as well as database searches suggested the presence of heterogeneous first exons for Mid1 as well as alternative promoters in addition to the retroviral LTR. To investigate the expression pattern of the chimeric transcript and to determine the contribution of the retroviral promoter in driving the expression of Mid1 transcripts, we performed real-time quantitative reverse transcription–polymerase chain reaction (RT-PCR) on cDNA from placenta and various fetal tissues because preliminary data suggested that the level of chimeric transcripts was highest in those tissues. Amplifications were carried out using a chimeric transcript–specific primer pair (primers 36 and 47) to detect Mid1 mRNAs derived from the retroviral element. Additional PCR with primers from a region of Mid1 common to all isoforms (primers 48 and 49) and from GAPDH were also used to assess the total level of Mid1 transcripts originating from all promoters as well as the quality of the RNA. The real-time RT-PCR data revealed that the LTR promoter was tissue specific. Chimeric mRNAs were found to constitute a significant proportion of total Mid1 transcripts in placenta and fetal kidney, where they represented 25% and 22% of overall Mid1 mRNAs, respectively (see fig. 2A ). Further analysis on placental sections indicated high LTR promoter activity in chorion, decidua, and villi where 24%, 16%, and 38%, respectively, of Mid1 transcripts contained the retroviral 5′ UTR variant (fig. 2B ).
Functional Analysis of the Retroviral Promoter
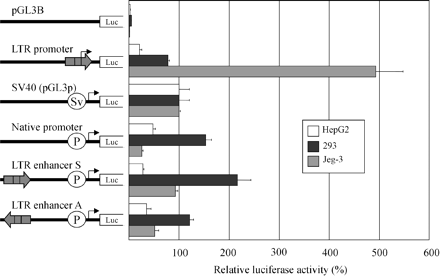
To establish that the putative Mid1 retroviral promoter was functional, a reporter construct containing the LTR was transfected into human choriocarcinoma cell line Jeg-3, embryonic kidney cell line 293, and liver cell line HepG2. Concordant with the real-time chimeric expression results, the data obtained with the transient transfections suggest that the Mid1 HERV-E LTR is a powerful promoter in placenta and embryonic kidney (see fig. 3 ). Indeed, in the placental cell line Jeg-3, the activity of the LTR was five times stronger than that of the SV40 promoter, whereas in the embryonic kidney cell line, the LTR was nearly as strong as SV40. In the liver cell line, however, the LTR had much weaker activity, suggesting tissue specificity in the LTR promoter.
We generated additional luciferase reporter constructs to examine whether the retroviral sequence could also provide functional enhancing activity to an adjacent alternative Mid1 promoter. Because no functional studies have been performed to date on Mid1 promoters, we cloned a 1.1-kb region upstream of the nonretroviral Mid1 first exon present under accession number Y13667 (the cloned sequence represents nucleotides 50444–51575 under accession number AC079314.26). We tested the promoter activity of this region in the above three cell lines and then determined whether the presence of the LTR upstream of this Mid1 promoter influenced expression level of the reporter gene. As shown in figure 3 , this region was shown to have strong promoter activity in 293 and weaker activity in Jeg-3 and HepG2. Interestingly, these experiments suggested that the native promoter activity was somewhat enhanced in Jeg-3 cells when the LTR was inserted upstream of the native promoter in either orientation. In addition, we also determined (results not shown) that the LTR enhanced the activity of the SV40 promoter when inserted in either orientation. These experiments suggest that the LTR plays a functional cellular role in both placenta and embryonic kidney by acting as an alternative promoter and possibly also by enhancing the activity of the nonretroviral promoter.
Deletion Study of the Retroviral Promoter
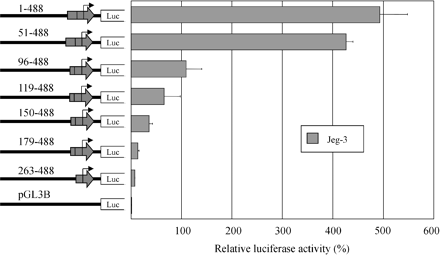
To further characterize the retroviral promoter, we generated a series of 5′ deletion constructs of the LTR. Because the LTR appears to be well used in placenta, we conducted our transfections in the placental cell line Jeg-3. As shown in figure 4 , successive deletions from the 5′ end of the LTR resulted in a continuous decrease in promoter activity, suggesting the presence of several positive regulatory elements upstream of the TATA box, which is located at position 352 of the LTR. A fourfold difference in promoter activity was observed between construct 51-488 and 96-488, suggesting a role for the region between positions 51 and 96 of the LTR (see fig. 1 ) in conferring high promoter activity. Examination of this section of the LTR revealed the presence of several transcription factor binding sites including GATA1, members of the ETS-type family, interferon response factors, C/EBP, AP2, and Sp1. But no consensus binding sites were identified in that region for placenta or embryonic kidney–specific transcription factors (or for both), suggesting that another region of the LTR confers tissue specificity to the LTR, whereas positions 51–96 enable high promoter activity.
Analysis of the Integration Time and Potential Impact of the Retroviral Element
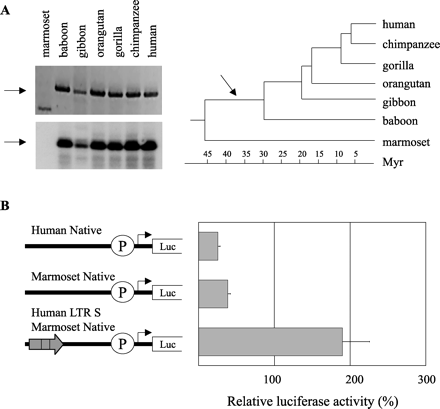
We performed PCR on genomic DNA from various primates to determine the evolutionary age of the Mid1 HERV-E element. Using a sense primer located upstream of the retroviral integration site in humans as well as an antisense primer present within the 5′ LTR of the HERV-E element, we confirmed the presence of the retroviral element in human, chimpanzee, gorilla, orangutan, gibbon, and baboon but were unable to amplify the element in marmoset (a New World Monkey) (see fig. 5A ). We believe that the absence of amplified retroviral product in marmoset indicates the lack of the HERV-E element at that location and not the result of rearrangement in that region because we were able to amplify nearby marmoset sequence upstream as well as downstream of the orthologous retroviral integration site. Because New World Monkeys diverged from Old World Monkeys (baboon) approximately 30–40 MYA (Sibley and Ahlquist 1987 ), our results suggest that the HERV-E in the Mid1 locus integrated over 30 MYA. Comparison of the 5′ and 3′ LTRs of the Mid1 HERV-E element revealed that they are 93% identical. If one assumes a mutation rate of 0.15%–0.21% per million years (Li and Tanimura 1987 ; Tristem 2000 ), this level of LTR identity roughly agrees with the integration time obtained from the genomic PCR studies.
To try to assess the possible regulatory impact of the retroviral element at the time of integration, we decided to measure the effect of the HERV-E LTR on the nonretroviral Mid1 promoter of a species which lacks the HERV-E LTR in the Mid1 locus. We therefore generated a Mid1 luciferase construct containing the marmoset Mid1 region which is orthologous to the human 1.1-kb native promoter tested above. The marmoset region was amplified using the human primers 12 and 70 because the marmoset region had not been sequenced. Sequencing of the marmoset segment revealed that it was very similar to the human region (90% identity). We then inserted the human LTR upstream of the marmoset segment in the reporter construct to determine whether the LTR would affect marmoset promoter activity. The transfection data, as shown in figure 5B, suggest that the marmoset Mid1 native promoter has slightly stronger promoter activity than the human native promoter in Jeg-3 cells. In addition, the insertion of the human LTR upstream of the native marmoset promoter enhances its activity fivefold, suggesting that the LTR can also act as a transcriptional enhancer of the Mid1 promoter in other species.
Discussion
In the present study, we have identified and characterized a hybrid transcript for the Mid1 gene. This chimeric mRNA originates from a retroviral LTR belonging to the HERV-E family and is spliced to the coding sequence of the Mid1 gene. We have established that the LTR is used as an alternative promoter and that differential use of the retroviral promoter generates a Mid1 mRNA isoform with a variant first exon. Although the fusion transcript possesses a different 5′ UTR, it would encode a Mid1 protein identical to the sequence deposited in Genbank (Y13667 and AF035360) by other groups (Quaderi et al. 1997 ; Perry et al. 1998 ).
In this report, we have provided the first description of a functional Mid1 promoter. We have demonstrated that an HERV-E LTR transcribes Mid1 in a tissue-specific fashion because high levels of chimeric transcripts were identified in placenta and embryonic kidney but not in other tissues. The evidence for this tissue specificity was strengthened by transient transfections of a luciferase construct containing the Mid1 HERV-E LTR in different cell lines. Consistent with the in vivo real-time PCR findings, the promoter activity of the LTR varied significantly depending on cell type but was found to be highest in placenta and embryonic kidney cell lines. In addition, the LTR was also shown to increase the activity of the nonretroviral Mid1 promoter in a placental cell line, suggesting a dual role for the Mid1 HERV-E LTR as both an alternative promoter and enhancer. Deletion experiments of the retroviral promoter indicated the presence of a positive regulatory region, necessary for high placental promoter activity, between positions 51 and 96 of the LTR. Although a number of potential transcription factor binding sites were found within this region, including possible sites for GATA1, members of the ETS-type family, interferon response factors, C/EBP, AP2, and Sp1, additional studies will be required to identify the transactivating element that enables the retroviral promoter to participate in the transcriptional regulation of Mid1.
The use of a retroviral LTR by Mid1 raises interesting questions regarding the functional significance of this alternative promoter. The use of multiple promoters and transcriptional start sites is believed to be an important evolutionary mechanism that provides flexibility in the regulatory control of gene expression (Ayoubi and Van De Ven 1996 ). Alternative promoter usage has been shown to be responsible for the tissue-specific or developmental stage–specific (or both) expression of a number of genes (Chretien et al. 1988 ; Borgese et al. 1993 ; Teerink et al. 1994 ; Anusaksathien et al. 2001 ). In several cases, the alternatively transcribed genes studied contained both a tissue-specific as well as a ubiquitous promoter (Ayoubi and Van De Ven 1996 ). The human porphobilinogen deaminase gene, for example, was found to be transcribed from both a housekeeping promoter as well as an alternative erythroid-specific promoter (Chretien et al. 1988 ). The expression of Mid1 is likely controlled by a similar transcriptional mechanism. Whereas our data indicate that the chimeric Mid1 isoform is transcribed in a tissue-specific fashion, others have reported that nonretroviral Mid1 transcripts are expressed ubiquitously (Quaderi et al. 1997 ; Perry et al. 1998 ). When combined, these results suggest that Mid1 is transcribed in all tissues from one or multiple nonretroviral promoters, whereas the retroviral LTR is responsible for the tissue-specific expression in placenta and embryonic kidney. It is as yet unclear how or whether LTR-driven expression of Mid1 in these two tissues contributes to the function of the gene or to the pathology of the Opitz syndrome. No specific abnormalities in kidney or placental tissues have been described for this genetic syndrome, which is characterized primarily by defects in midline structures such as cleft lip, heart problems, genitourinary abnormalities, laryngotracheal defects, and mental retardation (Robin, Opitz, and Muenke 1996 ). Because the LTR is responsible for a significant fraction of Mid1 transcripts in certain tissues, it is possible that Mid1 function could be affected by activity of the LTR, particularly if small changes in the level of this protein are phenotypically important. But the potential effects of moderate fluctuations in Mid1 levels have not been investigated.
The presence of a functional Mid1 promoter within a retroviral LTR is intriguing because HERV elements are found only in primate species. The insertion of the HERV-E element upstream of the Mid1 gene, 30–40 MYA as suggested by our study, may have contributed to the evolution of transcriptional regulatory elements controlling the expression of Mid1 in apes and Old World Monkeys but not in New World Monkeys or other mammals. Integration of this additional promoter could have resulted in increased overall expression of Mid1 in placenta and embryonic kidney. This hypothesis is supported by our observation that insertion of the retroviral LTR upstream of the marmoset native Mid1 promoter region, which does not naturally harbor the Mid1 HERV-E element, results in enhanced promoter activity in reporter gene assays. The higher levels of Mid1 transcripts might have resulted in a phenotype because the Mid1 protein appears to act in a dose-dependent fashion. For example, carrier females for Opitz syndrome who are heterozygous for Mid1 manifest some of the symptoms of the recessive disease (Quaderi et al. 1997 ; Brooks et al. 1998 ). Although it is difficult to assess the biological significance of increased Mid1 levels, one can assume that integration of the HERV-E was not detrimental to the species, or the element would not have been fixed in the population.
The number of documented cases in which retroviral sequences have been assimilated in the genome of various species to provide enhancers, promoters, or polyadenylation signals has grown rapidly in the last few years. One of the best characterized of these examples involves the human salivary amylase gene family which acquired a tissue-specific enhancer as a result of the integration of a retroviral element belonging to the HERV-E family (Samuelson et al. 1990 ; Ting et al. 1992 ). Other interesting cases are that of the apolipoprotein-C1 and endothelin-B receptor genes which have gained additional promoters as a result of the integration of retroviral sequences in the vicinity of the genes (Medstrand, Landry, and Mager 2001 ). Interestingly, the HERVs that contribute alternative promoters to these cellular genes as well as the element that has been reported to influence the expression of the pleiotrophin gene (Schulte et al. 1996 ) belong to the HERV-E retroviral family. In addition, we have recently discovered through database searches that a solitary HERV-E LTR situated on chromosome 16q24 (accession NT_010542) with very high identity (93%) to the Mid1 5′ LTR is also involved in cellular gene regulation by serving as a polyadenylation signal for the human P-R domain zinc finger protein (PRDM7) mRNA (LocusLinkID 11105) (unpublished data). Although HERV-E elements are not the most abundant retroviral group in the genome (Wilkinson, Mager, and Leong 1994 ), they appear to play a predominant role in contributing regulatory sequences to cellular genes. HERV-E sequences might be located in the vicinity of genes more frequently than other retroviral elements, increasing their probability of contributing to cellular gene regulation. Alternatively, it is possible that a significant fraction of HERV-E LTRs still harbor intact promoter or enhancer elements (or both) or that they are less subject to transcriptional shutdown by methylation or other mechanisms. But determination of the characteristics that facilitate the involvement of HERV-E elements in the regulation of cellular genes awaits future research.
In summary, the studies reported here demonstrate that an HERV-E LTR acts as an alternative tissue-specific promoter for the Opitz syndrome gene Mid1 and suggest a biological role for some retroelements in the transcriptional regulation of human genes.
William Jeffery, Reviewing Editor
Keywords: human endogenous retroviruses retroelements alternative promoters gene regulation Opitz syndrome Mid1
Address for correspondence and reprints: Dixie L. Mager, Terry Fox Laboratory, British Columbia Cancer Agency, 601 West 10th Avenue, Vancouver, B.C., Canada V5Z 1L3. dixie@interchange.ubc.ca
Fig. 1.—Characterization of human retroviral Mid1 chimeric transcripts. (A) The position of the HERV-E retroviral element, depicted as a rectangle flanked by two arrows, is shown with respect to the second exon of Mid1, represented by a box. The location of the putative TATA box is indicated in the LTR (arrow), and the splice donor site is indicated by SD. The HERV-E 5′ LTR is situated over 20 kb upstream of the second exon of Mid1. The native promoter is represented by a circle and is located at an undetermined distance upstream of the retroviral element. The translation initiation site is shown at the beginning of exon 2. Two alternative Mid1 cDNA isoforms are schematically illustrated below the diagram of the genomic locus. The retroviral first exon is denoted as 1R, and 1N indicates the native first exon reported previously (Quaderi et al. 1997 ). Both alternative first exons splice to the second exon using a common splice acceptor site at the beginning of exon 2. (B) Genomic sequence of the HERV-E element in the Mid1 locus (sequence represents positions 20480–19510 in accession AC079314.26). The 5′ LTR is enclosed by a dashed box, and the putative TATA box is shown in bold. The retroviral first exon sequence (on the basis of three longest 5′ RACE clones) is shown in uppercase and framed
Fig. 2.—Comparison of overall Mid1 and chimeric Mid1 expression levels. The relative abundance of Mid1 chimeric transcripts compared with overall Mid1 mRNA as measured by real-time PCR is shown. Total cDNAs from various human tissues were subjected to PCR using different sets of primers to detect either all Mid1 transcripts, chimeric Mid1, or GAPDH mRNA. Total Mid1 levels relative to GAPDH are depicted by bars. The black portion represents the percentage (written above the bars) of overall Mid1 mRNAs that possess retroviral first exons. The relative abundance of Mid1 isoforms in fetal tissues and placenta is illustrated in panel A, and the levels in placental sections are shown in panel B
Fig. 3.—Transient transfection analysis of promoter and enhancer activity of the retroviral LTR. The left part of the figure shows the various constructs used to transiently transfect the HepG2 human liver cell line (white bars), the 293 human embryonic kidney cell line (black bars), and the Jeg-3 human choriocarcinoma cell line (gray bars). The luciferase activities are corrected for transfection efficiency with the Renilla luciferase pRL-TK plasmid and are presented as percentages of the activity of the pGL3p vector. Each bar is the mean of the relative luciferase activity from at least two experiments ±SD
Fig. 4.—Deletion analysis of the retroviral LTR promoter. Representation of the 5′ LTR deletion plasmids transiently transfected to the Jeg-3 cell line. Numerical designation of each construct is based on assigning the first nucleotide of the LTR a position of 1. The plasmid 1-488 corresponds to the full-length LTR and is identical to the LTR promoter construct of figure 4 . The luciferase activities shown are corrected for transfection efficiency with the Renilla luciferase pRL-TK plasmid and are presented as percentages of the activity of the pGL3p vector. Each bar is the mean of the relative luciferase activity from at least two experiments ±SD
Fig. 5.—Evolutionary analysis of the HERV-E element. (A) Top left: an ethidium bromide–stained gel of Mid1 HERV-E LTR–specific products obtained from primate genomic DNA. Bottom left: hybridization to confirm the authenticity of the amplified products. Note that the PCR product from marmoset DNA is nonspecific because it does not hybridize. In both pictures, the arrows represent the expected sizes. Right: dendrogram illustrating the divergence time point of various primates. The arrow shows the proposed integration time of the HERV-E element in the Mid1 locus. (B) Schematic representation of plasmids used to transiently transfect the Jeg-3 human choriocarcinoma cell line (gray bars). The luciferase activities shown are corrected for transfection efficiency with the Renilla luciferase pRL-TK plasmid and are presented as percentages of the activity of the pGL3p vector. Each bar is the mean of the relative luciferase activity from at least two experiments ±SD
We thank members of our laboratory for helpful discussions and Christine Kelly for help with manuscript preparation. This work was supported by a grant from the Canadian Institutes of Health Research to D.L.M. with core support provided by the B.C. Cancer Agency. J.-R.L. is the recipient of a studentship from the Canadian Institutes of Health Research. P.M. was supported by a fellowship from the Knut and Alice Wallenburg Foundation.
References
Altschul S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, D. J. Lipman,
Anusaksathien O., C. Laplace, X. Li, Y. Ren, L. Peng, S. R. Goldring, D. L. Galson,
Ayoubi T. A., W. J. Van De Ven,
Baust C., W. Seifarth, U. Schon, R. Hehlmann, C. Leib-Mosch,
Bi S., O. Gavrilova, D.-W. Gong, M. M. Mason, M. Reitman,
Borgese N., A. D'Arrigo, M. De Silvestris, G. Pietrini,
Brooks J. K., C. O. Leonard, J. K. Zawadzki, A. K. Ommaya, B. A. Levy, J. M. Orenstein,
Cainarca S., S. Messali, A. Ballabio, G. Meroni,
Chretien S., A. Dubart, D. Beaupain, N. Raich, B. Grandchamp, J. Rosa, M. Goossens, P. H. Romeo,
Cox T. C., L. R. Allen, L. L. Cox, B. Hopwood, B. Goodwin, E. Haan, G. K. Suthers,
Di Cristofano A. D., M. Strazzullo, L. Longo, G. La Mantia,
Gaudenz K., E. Roessler, N. Quaderi, et al. (12 co-authors)
Goodchild N. L., D. A. Wilkinson, D. L. Mager,
International Human Genome Sequencing Consortium.
Kazazian H. H. J., J. V. Moran,
Li W. H., M. Tanimura,
Liu J., T. D. Prickett, E. Elliott, G. Meroni, D. L. Brautigan,
Lower R., J. Lower, R. Kurth,
Medstrand P., J. R. Landry, D. L. Mager,
Medstrand P., M. Lindeskog, J. Blomberg,
Perry J., S. Feather, A. Smith, S. Palmer, A. Ashworth,
Quaderi N. A., S. Schweiger, K. Gaudenz, et al. (16 co-authors)
Robin N. H., J. M. Opitz, M. Muenke,
Samuelson L. C., K. Wiebauer, C. M. Snow, M. H. Meisler,
Schulte A. M., S. Lai, A. Kurtz, F. Czubayko, A. T. Riegel, A. Wellstein,
Schweiger S., J. Foerster, T. Lehmann, et al. (12 co-authors)
Sibley C. G., J. E. Ahlquist,
Teerink H., M. A. Kasperaitis, C. H. De Moor, H. O. Voorma, A. A. Thomas,
Ting C. N., M. P. Rosenberg, C. M. Snow, L. C. Samuelson, M. H. Meisler,
Tristem M.,
Trockenbacher A., V. Suckow, J. Foerster, J. Winter, S. Krauss, H. H. Ropers, R. Schneider, S. Schweiger,
Van den Veyver I. B., T. A. Cormier, V. Jurecic, A. Baldini, H. Y. Zoghbi,
Wilkinson D. A., J. D. Freeman, N. L. Goodchild, C. A. Kelleher, D. L. Mager,