-
PDF
- Split View
-
Views
-
Cite
Cite
Juliane K. Unger, Claudia Haltern, Bernd Dohmen, Axel Gressner, Christian Grosse-Siestrup, David A. Groneberg, Rolf Rossaint, Albumin and hydroxyethyl starch 130 kDa/0.4 improve filter clearance and haemocompatibility in haemo- and plasmafiltration—an in vitro study, Nephrology Dialysis Transplantation, Volume 20, Issue 9, September 2005, Pages 1922–1931, https://doi.org/10.1093/ndt/gfh913
Close - Share Icon Share
Abstract
Background. Apart from their standard applications, haemofiltration (HF) and plasmafiltration (PF) may provide helpful therapy for sepsis, multiple organ- and acute liver-failure. Some colloids cause either decreases or increases in blood cell agglomeration. We hypothesized that solutions which reduce cell aggregability may lead to both improved filter clearance and better haemocompatibility due to decreasing rates of clogged hollow fibres.
Methods. Heparinized porcine blood (5 IU/ml) was used in an in vitro circuit. The filter types tested were from GAMBRO: HF66D (effective membrane surface: 0.6 m2) and PF1000N (effective membrane surface: 0.15 m2). Albumin (ALB), hydroxyethyl starch (HES) 200/0.5, HES 130/0.4, gelatin (GEL) or normal saline (0.9%) were added to the blood (n = 6/group). Recirculation systems were run for 2 h. Spontaneous haemolysis and filter resistance >420 mmHg were selected as indications of maximal flow rates. Sieving coefficients were determined for 17 parameters at the lowest and highest blood flows and filtration rate.
Results. Based on the filter types used, supplementation of ALB and HES130/0.4 led to an improved filter clearance without increasing the number of clogged capillary membranes or causing impaired haemocompatibility. Sieving coefficients for most solutes were independent of volume substitute and flow rate. Haemocompatibility and filter clearance deteriorated after addition of HES200 or GEL to the blood.
Conclusions. Under standardized in vitro conditions, we found that colloids which reduce cell aggregability cause improved HF- and PF-performance. This phenomenon may provide new options for higher clearances and may lead to new concepts in low dose anticoagulation.
Introduction
The development of high quality membranes for blood purification has provided good biocompatibility and created various sieving characteristics for haemofiltration (HF) and plasmafiltration (PF). Plasma- and haemofilters have been specifically engineered to offer filtration devices that allow low- to middle-range clearance rates. Nonetheless, they have been used without modifications for both high volume exchange [1,2] and for recirculating extracorporeal detoxification systems [3]. Contact with artificial surfaces leads to the stimulation of cell adhesion and further activates the coagulation system, which are both associated with membrane clotting in the hollow fibres within the filters. Because of these findings, biocompatibility issues are thought to be the primary cause of blocked capillary membranes in HF- and PF-modules. As a consequence, interest in utilizing HF and PF for complex extracorporeal detoxification strategies has led to ongoing efforts to provide improved regimes for anticoagulation [4–6]. However, we have found that the simple aggregation of erythrocytes may provide an additional key factor for the development of blocked capillaries (membrane clogging). Once fibres are blocked, a vicious cycle is initiated through an increase in transmembrane pressure, deteriorated filter clearance and reduced haemocompatibility [7,8]. Under in vitro conditions, a lowered albumin:globulin ratio was found to impair the performance of PF when compared to settings based on a normalized albumin:globulin ratio. To explain these findings, a lowered albumin:globulin ratio is associated with higher cell aggregability. Interestingly, we found that the filtration deterioration associated with the lowered albumin:globulin ratio could be reversed by two completely different experimental strategies: an increase in heparin dose and supplementation with albumin [7]. Because HF and PF are based on convective transport mechanisms, plasma volume inside the hollow fibres is reduced due to pressure gradient-driven filtration. The latter leads to a higher density of cell count per volume as well as to a higher plasma protein concentration in case of HF. Thus, the intrinsic tendency towards cell- and protein-agglomeration, which is triggered by individual patient blood- and plasma-composition [9,10], becomes of great importance. As a consequence, pathophysiologically changed blood composition in patients scheduled for treatment with an extracorporeal detoxification system may lead to deteriorated blood rheology associated with increased cell agglomeration inside the hollow fibres of the filters.
Thus, we examined whether protective effects caused by correction of the albumin:globulin ratio reproducibly results in reduced cell agglomeration. Since certain colloids have been described to improve blood rheology, we performed a prospective in vitro study to assess the impact of the most common colloidal solutions on overall filtration performance, including filter clearance and haemocompatibility.
Subjects and methods
Study protocol
Based on the principle of a prospective, randomized, controlled study design, we selected five volume substitutes that included normal saline (SAL) (NaCl 0.9%), albumin (ALB), gelatin (GEL) (gelatin polysuccinate, middle molecular weight of 30 kDa), and two types of hydroxyethyl starch preparations: middle molecular weight of 200 kDa with a degree of substitution of 0.5 HES200/0.5 (HES200) and a middle molecular weight of 130 kDa with a degree of substitution of 0.4, HES130/0.4 (HES130). Substances were chosen because of their different influences on blood rheology and their importance in intensive care medicine and volume resuscitation. Although use of these colloids is controversial because of varying adverse effects, such as risk of renal failure, bleeding, deterioration of pulmonary function, and allergic reactions, they are the most commonly used colloids in Europe. HES130 is a newer type of colloid and knowledge about its impact on coagulation patterns and renal function is still scarce.
Study groups
Five different experimental groups were set for plasmafiltration (PF group 1–5) as well as for haemofiltration (HF group 1–5), and these included six experiments per group. (i) Group 1 (n = 6): porcine blood supplemented with SAL (NaCl 0.9%; B. Braun Melsungen, Melsungen, Germany). (ii) Group 2 (n = 6): porcine blood supplemented with ALB (20%®; Behringwerke, Marburg, Germany). (iii) Group 3 (n = 6): porcine blood supplemented with HES200 (Haes-steril® 10%; Fresenius Kabi Deutschland GmbH, Bad Homburg v.d.H., Germany). (iv) Group 4 (n = 6): porcine blood supplemented with HES130 (Volu-Ven® 6%; Fresenius Kabi Deutschland GmbH, Bad Homburg v.d.H., Germany). (v) Group 5 (n = 6): porcine blood supplemented with GEL (Gelafundin 4%®; B. Braun Melsungen, Melsungen, Germany).
The supplementation ratio of ml solution/ml blood was 1:20 (50 μl solution/ml blood). The percentage of ALB solution was 20% since this was required to adjust the low albumin:globulin ratio of 0.8 of porcine blood which is comparable to that of hypoalbumineuria in humans – to levels that were comparable to normal values in humans (1.2) [7,8]. Concentrations of GEL (4%) and HES130 (6%) were at given values set by the manufacturers. For HES200, a 10% solution was chosen to provide both an intermediary concentration (ALB vs GEL and HES130) and to imitate situations during which dose limits are achieved in clinical application.
Parameters investigated
(i) Pressure profiles: pressure before the filter (arterial pressure, Pa), venous and filtrate pressure (Pv, Pf), transmembrane pressure (TMP) and pressure drop (Pa-Pv). (ii) Highest blood and filtration rates (Qb/Qf) that could be achieved. (iii) Sieving coefficients during PF: total protein, fibrinogen, LDH, albumin,
Thresholds for determination of maximal flow rates
We had two different variations of highest possible flow rates: (i) highest flow rate that could be run under pressure control; and (ii) highest flow rate that could be performed without spontaneous haemolysis.
Materials and assays used for laboratory analysis
Laboratory analyses
Blood, plasma and filtrate samples were analysed using a blood counter (Celltek m; Bayer Vital GmbH & Co KG, Munic, Germany), and an ABL 510 and an EML 100 (Radiometer; Copenhagen and Denmark Vettest 8008, IDEXX GmbH, Wörrstadt, Germany). Haemolysis was determined from the content of free plasma haemoglobin (fHb) (cyanide-reaction; Diagnostica Merck, 9405, Darmstadt, Germany; UV photometer Ultraspec II; Biochrom LKB, Cambridge, UK) [7,8].
Plasma coagulation analysis
Extrinsic and intrinsic coagulation factors were analysed measuring prothrombin time and activated partial thromboplastin time. Activated partial thromboplastin time was analysed with Dade® ActinFS reagent, and prothrombin time with the Dade® Innovin reagent (both reagents: Dade Behring, Marburg, Germany). For the determination of fibrinogen concentrations, the method of Clauss and the Fibrinogen a® reagent (Diagnostica Stago, Boehringer, Mannheim, Germany) were used. Polybrene® (Abbott Laboratories, Dallas, USA) was added to the original ActinFS and Innovin reagents in order to antagonize the high heparin dosage [7,8].
Blocked capillaries
At the end of experiments, filters were rinsed with 3 l of SAL and with a 3% buffered formaldehyde solution (pH 7.4) using a flow rate of 200 ml/min. Thereafter, filters were cut open, the overall fibre bundle was visually examined and percentage of blocked capillaries was estimated by two independent observations. Representative membranes were sampled for standard histological preparation and haematoxiline–eosine staining was performed (Department of Pathologie, RWTH Aachen). Determinations between membrane clotting and membrane clogging were performed using light microscopy examinations.
Blood
Porcine blood
Porcine blood was collected at a slaughterhouse from healthy pigs using a standardized sterile collecting system primed with heparin to achieve anticoagulation with 5 IU/ml of blood. In accordance with the upper level of haematocrit (Hct) that may be found during the two different types of apheresis, an Hct of 40–45% was chosen for PF experiments and an Hct of 35–40% was set for HF experiments. Since in vitro systems based on whole blood require short protocols to avoid artifacts induced by deteriorating blood quality, high Hct levels were chosen to accelerate the vicious cycle of deterioration. The duration of the experiment from priming to final values was 2 h.
Apheresis
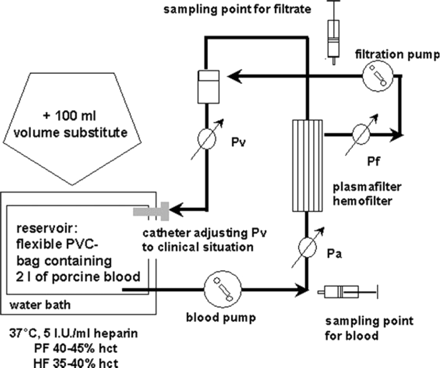
Extracorporeal in vitro circuit (Figure 1)
A closed in vitro circuit was assembled [7,8] using a clinically proven plasma filtration pump (AK10, Gambro Hechingen, Germany). Plasma- and haemofilters were PF1000N and HF66D from Ca. Gambro. Specifications of these filters are given in Table 1. Standard bloodlines were used. Arterial and venous lines were connected to a 2 l blood reservoir that was stored in a heated water bath (37°C). The outflow of the filtration line was connected to the venous bubble trap allowing recirculation mode for both blood and filtrate. Using the commercially installed electronic-transducers of the apheresis device (AK10®), pressure values were continuously measured. The average pressure inside the filtrate compartment (Pf, filtration pressure) was measured with additional control-transducers (Transpac; Abbott GmbH, Wiesbaden, Germany) connected to a multichannel recorder outside the apheresis monitor (model S 66; Hewlett Packard, Bad Homburg, Germany). While following the manufacturer recommendations for the filter types, the experimental protocol started with 125 ml/min blood flow (Qb) and with 15 ml/min filtrate flow (Qf) for PF. HF was started with a Qb/Qf of 75/15 ml/min. Pressures were documented every 20 min prior to blood- and filtrate sampling. Thereafter, flow rates were increased for another 20 min.
Principle of the in vitro circuit. Pa, Pv, TMP and Pf were measured as indicated according to the procedure of clinical application. Haemofiltration was performed for a total of 2 h for each experiment. Pa, arterial pressure; Pv, venous pressure; TMP, transmembrane pressure; Pf, average pressure inside the filtrate compartment.
Commercial specifications of the two filter types used in the present study
| Filter type . | Plasma filter PF1000N (Gambro) . | Haemofilter FH66D (Gambro) . |
|---|---|---|
| Effective membrane area (m2) | 0.15 | 0.6 |
| Fibre dimensions | ||
| Inner diameter (µm) | 330 | 220 |
| Maximum pore size (µm) | 0.5 | – |
| Effective length (mm) | 140 | 140 |
| Components | Materials | Materials |
| Membrane material | Polypropylene | Polyamid |
| Potting material | Polyurethane | Polyurethane |
| Housing | Polycarbonate | Polycarbonate |
| Filter type . | Plasma filter PF1000N (Gambro) . | Haemofilter FH66D (Gambro) . |
|---|---|---|
| Effective membrane area (m2) | 0.15 | 0.6 |
| Fibre dimensions | ||
| Inner diameter (µm) | 330 | 220 |
| Maximum pore size (µm) | 0.5 | – |
| Effective length (mm) | 140 | 140 |
| Components | Materials | Materials |
| Membrane material | Polypropylene | Polyamid |
| Potting material | Polyurethane | Polyurethane |
| Housing | Polycarbonate | Polycarbonate |
Commercial specifications of the two filter types used in the present study
| Filter type . | Plasma filter PF1000N (Gambro) . | Haemofilter FH66D (Gambro) . |
|---|---|---|
| Effective membrane area (m2) | 0.15 | 0.6 |
| Fibre dimensions | ||
| Inner diameter (µm) | 330 | 220 |
| Maximum pore size (µm) | 0.5 | – |
| Effective length (mm) | 140 | 140 |
| Components | Materials | Materials |
| Membrane material | Polypropylene | Polyamid |
| Potting material | Polyurethane | Polyurethane |
| Housing | Polycarbonate | Polycarbonate |
| Filter type . | Plasma filter PF1000N (Gambro) . | Haemofilter FH66D (Gambro) . |
|---|---|---|
| Effective membrane area (m2) | 0.15 | 0.6 |
| Fibre dimensions | ||
| Inner diameter (µm) | 330 | 220 |
| Maximum pore size (µm) | 0.5 | – |
| Effective length (mm) | 140 | 140 |
| Components | Materials | Materials |
| Membrane material | Polypropylene | Polyamid |
| Potting material | Polyurethane | Polyurethane |
| Housing | Polycarbonate | Polycarbonate |
Priming of the system
The in vitro circuit was rinsed with 2 l of SAL. Addition of volume substitutes to the blood was performed. Exchange of the preload with SAL against supplemented blood was performed in single pass mode wasting effluent as long as it still contained rinsing solution. Thereafter, blood was pumped from the collecting bag via the filter into the in vitro system. The recirculation mode was initiated when the scheduled system volume of 2 l of blood was achieved.
Statistical analyses
Because normal distributions with n = 6/group were not expected, we used nonparametric tests for further statistical analyses. Statistical comparison of the groups was performed using the Mann–Whitney U-test for unpaired samples. Analyses of haemocompatibility parameters to compare baseline values with values at the end of the experiment were performed using the Wilcoxon Rank Sum Test for paired samples. A probability value of P<0.05 was designated to indicate statistical significance. Analyses were performed using NCSS 2000.
Results
Comparison of initial blood composition
Statistical analyses of baseline values for Hct, leukocytes, thrombocytes, fibrinogen and ATIII revealed sporadic differences (Table 2). HES130 groups had lower initial fibrinogen concentrations for HF experiments, while fibrinogen values tended to be highest in PF experiments. In PF experiments, the HES130 group had higher platelet counts than HES200, but the leukocyte count was still lower than in the SAL-PF group. Basic conditions for anticoagulation with heparin were similar due to comparable ATIII-levels.
Baseline values of important blood composition parameters
| . | Hct (%) . | Platelets (×103/μl) . | Leukocytes (×103/μl) . | Fibrinogen (g/l) . | ATIII (%) . |
|---|---|---|---|---|---|
| PF groups | |||||
| SAL | 40±4 | 316±71 | 17±3a | 2.0±0.4 | 82±5 |
| ALB | 41±2 | 270±43 | 14±3 | 2.0±0.5 | 90±10 |
| HES200 | 40±5 | 234±29b | 17±7 | 2.2±0.4 | 91±7 |
| HES130 | 41±2 | 318±28b | 13±2a | 2.3±0.3 | 85±7 |
| GEL | 40±2 | 302±43 | 14±1 | 2.2±0.3 | 79±6 |
| HF groups | |||||
| SAL | 37±7 | 316±80 | 14±4 | 2.1±0.5 | 87±12 |
| ALB | 40±3 | 367±67 | 15±3 | 2.4±0.2 | 89±11 |
| HES200 | 38±4 | 332±76 | 16±6 | 2.1±0.3 | 92±12 |
| HES130 | 37±4 | 276±27 | 13±3 | 1.8±0.1c | 89±11 |
| GEL | 39±5 | 390±97 | 17±6 | 2.3±0.6 | 81±4 |
| . | Hct (%) . | Platelets (×103/μl) . | Leukocytes (×103/μl) . | Fibrinogen (g/l) . | ATIII (%) . |
|---|---|---|---|---|---|
| PF groups | |||||
| SAL | 40±4 | 316±71 | 17±3a | 2.0±0.4 | 82±5 |
| ALB | 41±2 | 270±43 | 14±3 | 2.0±0.5 | 90±10 |
| HES200 | 40±5 | 234±29b | 17±7 | 2.2±0.4 | 91±7 |
| HES130 | 41±2 | 318±28b | 13±2a | 2.3±0.3 | 85±7 |
| GEL | 40±2 | 302±43 | 14±1 | 2.2±0.3 | 79±6 |
| HF groups | |||||
| SAL | 37±7 | 316±80 | 14±4 | 2.1±0.5 | 87±12 |
| ALB | 40±3 | 367±67 | 15±3 | 2.4±0.2 | 89±11 |
| HES200 | 38±4 | 332±76 | 16±6 | 2.1±0.3 | 92±12 |
| HES130 | 37±4 | 276±27 | 13±3 | 1.8±0.1c | 89±11 |
| GEL | 39±5 | 390±97 | 17±6 | 2.3±0.6 | 81±4 |
SEM±SD, (P<0.05).
aSignificant difference between the groups.
bSignificant difference between the groups.
cSignificant difference from ALB, GEL, HES200 groups.
Baseline values of important blood composition parameters
| . | Hct (%) . | Platelets (×103/μl) . | Leukocytes (×103/μl) . | Fibrinogen (g/l) . | ATIII (%) . |
|---|---|---|---|---|---|
| PF groups | |||||
| SAL | 40±4 | 316±71 | 17±3a | 2.0±0.4 | 82±5 |
| ALB | 41±2 | 270±43 | 14±3 | 2.0±0.5 | 90±10 |
| HES200 | 40±5 | 234±29b | 17±7 | 2.2±0.4 | 91±7 |
| HES130 | 41±2 | 318±28b | 13±2a | 2.3±0.3 | 85±7 |
| GEL | 40±2 | 302±43 | 14±1 | 2.2±0.3 | 79±6 |
| HF groups | |||||
| SAL | 37±7 | 316±80 | 14±4 | 2.1±0.5 | 87±12 |
| ALB | 40±3 | 367±67 | 15±3 | 2.4±0.2 | 89±11 |
| HES200 | 38±4 | 332±76 | 16±6 | 2.1±0.3 | 92±12 |
| HES130 | 37±4 | 276±27 | 13±3 | 1.8±0.1c | 89±11 |
| GEL | 39±5 | 390±97 | 17±6 | 2.3±0.6 | 81±4 |
| . | Hct (%) . | Platelets (×103/μl) . | Leukocytes (×103/μl) . | Fibrinogen (g/l) . | ATIII (%) . |
|---|---|---|---|---|---|
| PF groups | |||||
| SAL | 40±4 | 316±71 | 17±3a | 2.0±0.4 | 82±5 |
| ALB | 41±2 | 270±43 | 14±3 | 2.0±0.5 | 90±10 |
| HES200 | 40±5 | 234±29b | 17±7 | 2.2±0.4 | 91±7 |
| HES130 | 41±2 | 318±28b | 13±2a | 2.3±0.3 | 85±7 |
| GEL | 40±2 | 302±43 | 14±1 | 2.2±0.3 | 79±6 |
| HF groups | |||||
| SAL | 37±7 | 316±80 | 14±4 | 2.1±0.5 | 87±12 |
| ALB | 40±3 | 367±67 | 15±3 | 2.4±0.2 | 89±11 |
| HES200 | 38±4 | 332±76 | 16±6 | 2.1±0.3 | 92±12 |
| HES130 | 37±4 | 276±27 | 13±3 | 1.8±0.1c | 89±11 |
| GEL | 39±5 | 390±97 | 17±6 | 2.3±0.6 | 81±4 |
SEM±SD, (P<0.05).
aSignificant difference between the groups.
bSignificant difference between the groups.
cSignificant difference from ALB, GEL, HES200 groups.
Pressure values and maximal flow rates
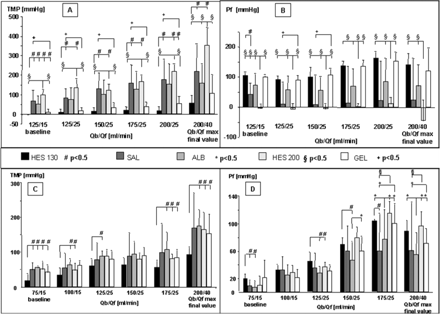
In PF (Figure 2A and B), differences in TMP were mainly triggered by differences in Pf. Supplementation of HES130 enabled the highest Pf values (and thus net filtrate flux) accompanied by the lowest range. All other substitutes demonstrated a higher range in Pf values and a tendency for lower net filtrate flux that was lowest in the HES200- and SAL-groups.
Pressure profiles of PF and HF. (A) TMP values in PF, (B) Pf values in PF, (C) TMP values in HF, (D) Pf values in HF. Pf was monitored in addition to the standard procedure during clinical application. Data are given as means + SD. Symbols indicate significance, P<0.05.
In HF (Figure 2C and D), supplementation of HES130 again led to high Pf values, indicating a high net filtrate flux, which was also reflected by significantly lower TMP values. In contrast, SAL- and ALB-supplementation led to lower rates of net filtrate flux resulting in highest TMP levels.
Membrane clogging and clotting
The PF groups HES130 and ALB both demonstrated a low number (18% mean) and range (5–30%) of blocked capillaries. These values were one-third lower than that found for GEL and SAL groups [GEL group: 30–90% (range) with a 67% mean; SAL-group: 40–80% (range) with a 50% mean]. When HES200 was added to the blood, the number of blocked capillaries at the end of the experiment increased to 80–95% (range) with an 88% mean. When examining both high filtrate flux and blocked capillaries, HES130 and ALB groups demonstrated the best performance.
During HF, the observed number of blocked capillaries led to more homogenous results. Filters operated with SAL-or ALB-supplemented blood showed about 50% of blocked hollow fibres. HES130-supplementation led to a rate of about 60%, while HES200- and GEL-supplementation led to further increases in blocked capillaries up to 70–75%. Thus, in HF higher net filtrate flux was accompanied by a higher percentage of blocked capillaries at the end of experiment and vice versa. The HES130-group performed best in terms of relation of filtrate flux and amount of blocked hollow fibres.
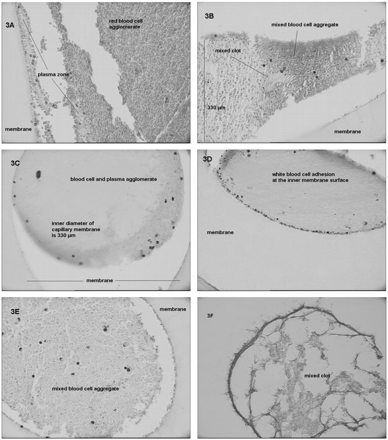
Analyses based on light microscopic investigations (Figure 3) revealed that the composition of cell aggregates differed depending on the type of volume substitute. In the case of SAL (Figure 3A) and ALB (Figure 3B) supplementation, microscopic pictures were comparable. However, aggregates clogging the hollow fibre during ALB supplementation contained a more homogenous distribution of residual plasma volume between the cells than during SAL supplementation. Capillaries obtained from PF operated during GEL supplementation (Figure 3C) demonstrated blood cell aggregates in which it was impossible to identify single cells. Packed blood cells and residual plasma proteins formed agglomerates. Furthermore, when compared to histology findings from the other study groups, an increase in white blood cell adhesion was found during GEL supplementation (Figure 3D). In the case of HES supplementation (Figure 3E), blood cell aggregates were again more comparable to the results obtained from natural substitutes (SAL, ALB). In HES130 group samples, coagulation was repeatedly associated with visually large, sharp cut molecules that were not found in histology samples from other study groups showing mixed clots (Figure 3F).
(A–F) Light microscopy pictures of PF capillary membranes of different study groups. (A, SAL-group; B, ALB-group; C, D GEL-group; E and F, HES130-group). Histology made from hollow fibres of PF, formaldehyde fixation, haematoxiline–eosine staining; magnification 400× and 600× (3F), respectively.
Haemocompatibility and coagulation features
Functional coagulation tests
The tests for functional coagulation, which included activated partial thromboplastin time and prothrombin time, did not show significant changes during the time course of the experiment in any of the groups. Fibrinogen concentrations were also stable, except for HF experiments with HES130 supplementation. In the HES130 HF group, fibrinogen concentrations increased [from 1.8±0.1 g/l to 2.1±0.1 g/l (mean±SD)]. This increase was above the theoretical value that was calculated for the method-associated side effect of plasma concentration, which is due to the removal of plasma water during filtrate sampling.
Haemolysis
With respect to haemocompatibility, supplementation of ALB enabled both filtration procedures (PF and HF) without significantly increasing fHb. This was in contrast to the results obtained with all the other PF and HF experiments, which were accompanied by significant haemolysis [increase of means for fHb: plasmafiltration HES200: 42 mg%, SAL: 10 mg%; GEL: 4 mg%, HES130: 2 mg%; haemofiltration SAL: 26 mg%; HES130: 15 mg%, HES200: 11 mg%, GEL: 10 mg%].
Leukocyte and platelet counts
We found conflicting leukocyte and platelet count results when comparing the two different types of filtration modes, PF and HF. During PF, HES130 supplementation led to a significant decrease in leukocyte counts. GEL supplementation led to a decrease in both leukocyte and platelet counts. Neither ALB nor SAL supplementation were accompanied by reductions in these two cell populations. During HF experiments, there were more effects on blood cell compatibility. The HES preparations and GEL caused decreases in either leukocyte or platelet counts. Experiments based on ALB and SAL supplementation demonstrated significant reductions in both leukocyte and platelet counts.
Sieving coefficients
The results for sieving coefficients are shown in Tables 3 and 4.
Sieving coefficients during plasma filtration
| . | Baseline (125/25) . | . | . | . | . | Qb/Qf max (200/40) . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SAL . | ALB . | HES200 . | HES130 . | GEL . | SAL . | ALB . | HES200 . | HES130 . | GEL . | ||||||||
| Total protein | 1.02±0.08 | 0.96±0.08 | 1.03±0.06 | 0.95±0.03 | 0.95±0.03 | 0.97±0.04 | 0.97±0.07 | 1.05±0.02 | 0.96±0.01 | 0.94±0.03 | ||||||||
| + | + | § | +*§ | * | + | |||||||||||||
| LDH | 0.96±0.08 | 0.92±0.15 | 0.75±0.32 | 0.9±0.18 | 1.0±0.04 | 0.97±0.24 | 0.9±0.11 | 0.94±0.09 | 0.94±0.09 | 0.96±0.04 | ||||||||
| Albumin | 1.03±0.08 | 0.89±0.1 | 1.09±0.03 | 0.92±0.04 | 0.95±0.06 | 0.99±0.04 | 0.95±0.02 | 1.0±0.02 | 0.94±0.02 | 0.91±0.02 | ||||||||
| * # | + * | + * § | § # | * | § % 1$ | + * § | * # & | & $ | + # % | |||||||||
| Fibrinogen | 1.03±0.09 | 1.07±0.07 | 1.17±0.15 | 1.04±0.05 | 1.03±0.07 | 1.05±0.05 | 1.05±0.05 | 1.06±0.06 | 1.08±0.04 | 1.06±0.04 | ||||||||
| Glucose | 1.04±0.16 | 1.00±0.07 | 0.76±0.30 | 0.99±0.02 | 1.03±0.03 | 1.02±0.02 | 1.02±0.03 | 1.00±0.01 | 1.0±0.01 | 0.99±0.01 | ||||||||
| + | + | + | + | |||||||||||||||
| Lactate | 0.99±0.11 | 0.94±0.07 | 0.91±0.17 | 0.91±0.09 | 0.91±0.14 | 0.99±0.01 | 0.98±0.02 | 0.97±0.05 | 0.97±0.05 | 0.89±0.14 | ||||||||
| * | + | + * | ||||||||||||||||
\(\mathrm{NH}_{\mathrm{4}}^{{+}}\) | 0.69±0.22 | 0.86±0.17 | 0.75±0.1 | 0.8±0.5 | 0.90±0.07 | 0.86±0.34 | 0.95±0.27 | 0.80±0.21 | 0.96±0.04 | 0.88±0.07 | ||||||||
| + | + | + | + | |||||||||||||||
| . | Baseline (125/25) . | . | . | . | . | Qb/Qf max (200/40) . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SAL . | ALB . | HES200 . | HES130 . | GEL . | SAL . | ALB . | HES200 . | HES130 . | GEL . | ||||||||
| Total protein | 1.02±0.08 | 0.96±0.08 | 1.03±0.06 | 0.95±0.03 | 0.95±0.03 | 0.97±0.04 | 0.97±0.07 | 1.05±0.02 | 0.96±0.01 | 0.94±0.03 | ||||||||
| + | + | § | +*§ | * | + | |||||||||||||
| LDH | 0.96±0.08 | 0.92±0.15 | 0.75±0.32 | 0.9±0.18 | 1.0±0.04 | 0.97±0.24 | 0.9±0.11 | 0.94±0.09 | 0.94±0.09 | 0.96±0.04 | ||||||||
| Albumin | 1.03±0.08 | 0.89±0.1 | 1.09±0.03 | 0.92±0.04 | 0.95±0.06 | 0.99±0.04 | 0.95±0.02 | 1.0±0.02 | 0.94±0.02 | 0.91±0.02 | ||||||||
| * # | + * | + * § | § # | * | § % 1$ | + * § | * # & | & $ | + # % | |||||||||
| Fibrinogen | 1.03±0.09 | 1.07±0.07 | 1.17±0.15 | 1.04±0.05 | 1.03±0.07 | 1.05±0.05 | 1.05±0.05 | 1.06±0.06 | 1.08±0.04 | 1.06±0.04 | ||||||||
| Glucose | 1.04±0.16 | 1.00±0.07 | 0.76±0.30 | 0.99±0.02 | 1.03±0.03 | 1.02±0.02 | 1.02±0.03 | 1.00±0.01 | 1.0±0.01 | 0.99±0.01 | ||||||||
| + | + | + | + | |||||||||||||||
| Lactate | 0.99±0.11 | 0.94±0.07 | 0.91±0.17 | 0.91±0.09 | 0.91±0.14 | 0.99±0.01 | 0.98±0.02 | 0.97±0.05 | 0.97±0.05 | 0.89±0.14 | ||||||||
| * | + | + * | ||||||||||||||||
\(\mathrm{NH}_{\mathrm{4}}^{{+}}\) | 0.69±0.22 | 0.86±0.17 | 0.75±0.1 | 0.8±0.5 | 0.90±0.07 | 0.86±0.34 | 0.95±0.27 | 0.80±0.21 | 0.96±0.04 | 0.88±0.07 | ||||||||
| + | + | + | + | |||||||||||||||
SEM±SD, P <0.05, pairs of symbols (+. #. *. §. $. %) indicate significant differences between the two groups.
Sieving coefficients during plasma filtration
| . | Baseline (125/25) . | . | . | . | . | Qb/Qf max (200/40) . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SAL . | ALB . | HES200 . | HES130 . | GEL . | SAL . | ALB . | HES200 . | HES130 . | GEL . | ||||||||
| Total protein | 1.02±0.08 | 0.96±0.08 | 1.03±0.06 | 0.95±0.03 | 0.95±0.03 | 0.97±0.04 | 0.97±0.07 | 1.05±0.02 | 0.96±0.01 | 0.94±0.03 | ||||||||
| + | + | § | +*§ | * | + | |||||||||||||
| LDH | 0.96±0.08 | 0.92±0.15 | 0.75±0.32 | 0.9±0.18 | 1.0±0.04 | 0.97±0.24 | 0.9±0.11 | 0.94±0.09 | 0.94±0.09 | 0.96±0.04 | ||||||||
| Albumin | 1.03±0.08 | 0.89±0.1 | 1.09±0.03 | 0.92±0.04 | 0.95±0.06 | 0.99±0.04 | 0.95±0.02 | 1.0±0.02 | 0.94±0.02 | 0.91±0.02 | ||||||||
| * # | + * | + * § | § # | * | § % 1$ | + * § | * # & | & $ | + # % | |||||||||
| Fibrinogen | 1.03±0.09 | 1.07±0.07 | 1.17±0.15 | 1.04±0.05 | 1.03±0.07 | 1.05±0.05 | 1.05±0.05 | 1.06±0.06 | 1.08±0.04 | 1.06±0.04 | ||||||||
| Glucose | 1.04±0.16 | 1.00±0.07 | 0.76±0.30 | 0.99±0.02 | 1.03±0.03 | 1.02±0.02 | 1.02±0.03 | 1.00±0.01 | 1.0±0.01 | 0.99±0.01 | ||||||||
| + | + | + | + | |||||||||||||||
| Lactate | 0.99±0.11 | 0.94±0.07 | 0.91±0.17 | 0.91±0.09 | 0.91±0.14 | 0.99±0.01 | 0.98±0.02 | 0.97±0.05 | 0.97±0.05 | 0.89±0.14 | ||||||||
| * | + | + * | ||||||||||||||||
\(\mathrm{NH}_{\mathrm{4}}^{{+}}\) | 0.69±0.22 | 0.86±0.17 | 0.75±0.1 | 0.8±0.5 | 0.90±0.07 | 0.86±0.34 | 0.95±0.27 | 0.80±0.21 | 0.96±0.04 | 0.88±0.07 | ||||||||
| + | + | + | + | |||||||||||||||
| . | Baseline (125/25) . | . | . | . | . | Qb/Qf max (200/40) . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SAL . | ALB . | HES200 . | HES130 . | GEL . | SAL . | ALB . | HES200 . | HES130 . | GEL . | ||||||||
| Total protein | 1.02±0.08 | 0.96±0.08 | 1.03±0.06 | 0.95±0.03 | 0.95±0.03 | 0.97±0.04 | 0.97±0.07 | 1.05±0.02 | 0.96±0.01 | 0.94±0.03 | ||||||||
| + | + | § | +*§ | * | + | |||||||||||||
| LDH | 0.96±0.08 | 0.92±0.15 | 0.75±0.32 | 0.9±0.18 | 1.0±0.04 | 0.97±0.24 | 0.9±0.11 | 0.94±0.09 | 0.94±0.09 | 0.96±0.04 | ||||||||
| Albumin | 1.03±0.08 | 0.89±0.1 | 1.09±0.03 | 0.92±0.04 | 0.95±0.06 | 0.99±0.04 | 0.95±0.02 | 1.0±0.02 | 0.94±0.02 | 0.91±0.02 | ||||||||
| * # | + * | + * § | § # | * | § % 1$ | + * § | * # & | & $ | + # % | |||||||||
| Fibrinogen | 1.03±0.09 | 1.07±0.07 | 1.17±0.15 | 1.04±0.05 | 1.03±0.07 | 1.05±0.05 | 1.05±0.05 | 1.06±0.06 | 1.08±0.04 | 1.06±0.04 | ||||||||
| Glucose | 1.04±0.16 | 1.00±0.07 | 0.76±0.30 | 0.99±0.02 | 1.03±0.03 | 1.02±0.02 | 1.02±0.03 | 1.00±0.01 | 1.0±0.01 | 0.99±0.01 | ||||||||
| + | + | + | + | |||||||||||||||
| Lactate | 0.99±0.11 | 0.94±0.07 | 0.91±0.17 | 0.91±0.09 | 0.91±0.14 | 0.99±0.01 | 0.98±0.02 | 0.97±0.05 | 0.97±0.05 | 0.89±0.14 | ||||||||
| * | + | + * | ||||||||||||||||
\(\mathrm{NH}_{\mathrm{4}}^{{+}}\) | 0.69±0.22 | 0.86±0.17 | 0.75±0.1 | 0.8±0.5 | 0.90±0.07 | 0.86±0.34 | 0.95±0.27 | 0.80±0.21 | 0.96±0.04 | 0.88±0.07 | ||||||||
| + | + | + | + | |||||||||||||||
SEM±SD, P <0.05, pairs of symbols (+. #. *. §. $. %) indicate significant differences between the two groups.
Sieving coefficients during haemofiltration
| . | Baseline (75/15) . | . | . | . | . | Qb/Qf max (200/40) . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SAL . | ALB . | HES200 . | HES130 . | GEL . | SAL . | ALB . | HES200 . | HES130 . | GEL . | ||||||||
| Creatinine | 0.66±0.14 | 0.57±0.17 | 0.5±0.17 | 0.37±0.13 | 0.61±0.16 | 0.78±0.13 | 0.80±0.24 | 0.97±0.40 | 0.49±0.13 | 0.81±0.22 | ||||||||
| + # | * | * + # | § | * | # | * + # § | + | |||||||||||
| Urea | 0.96±0.06 | 0.82±0.21 | 0.74±0.16 | 0.84±0.04 | 0.88±0.06 | 0.97±0.04 | 0.99±0.10 | 0.92±0.09 | 0.96±0.04 | 0.94±0.05 | ||||||||
| * + | + | * | ||||||||||||||||
| Glucose | 0.96±0.11 | 0.85±0.19 | 0.53±0.17 | 0.78±0.10 | 1.05±0.22 | 0.94±0.10 | 0.93±0.10 | 0.85±0.12 | 0.88±0.09 | 0.94±0.03 | ||||||||
| % + | * | * # § $ | + § % | + # | ||||||||||||||
| Lactate | 0.91±0.04 | 0.90±0.08 | 0.75±0.15 | 0.85±0.09 | 0.82±0.07 | 0.99±0.06 | 0.95±0.01 | 0.95±0.03 | 0.94±0.04 | 0.95±0.02 | ||||||||
| * | + | + | * | |||||||||||||||
| Na+ | 0.97±0.02 | 0.95±0.01 | 0.97±0.01 | 0.96±0.01 | 0.95±0.00 | 0.97±0.03 | 0.94±0.02 | 0.95±0.01 | 0.95±0.01 | 0.95±0.01 | ||||||||
| * + | + § | * # | # § | * | * | |||||||||||||
| K+ | 0.89±0.10 | 0.88±0.09 | 0.77±0.12 | 0.88±0.07 | 0.86±0.06 | 1.00±0.02 | 0.89±0.03 | 0.91±0.04 | 0.94±0.03 | 0.90±0.10 | ||||||||
| + | + | |||||||||||||||||
| Ca++ | 0.79±0.14 | 0.82±0.17 | 0.81±0.12 | 0.90±0.09 | 0.91±0.03 | 1.03±0.04 | 0.79±0.23 | 0.88±0.18 | 0.91±0.04 | 0.96±0.06 | ||||||||
| * | * | |||||||||||||||||
| . | Baseline (75/15) . | . | . | . | . | Qb/Qf max (200/40) . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SAL . | ALB . | HES200 . | HES130 . | GEL . | SAL . | ALB . | HES200 . | HES130 . | GEL . | ||||||||
| Creatinine | 0.66±0.14 | 0.57±0.17 | 0.5±0.17 | 0.37±0.13 | 0.61±0.16 | 0.78±0.13 | 0.80±0.24 | 0.97±0.40 | 0.49±0.13 | 0.81±0.22 | ||||||||
| + # | * | * + # | § | * | # | * + # § | + | |||||||||||
| Urea | 0.96±0.06 | 0.82±0.21 | 0.74±0.16 | 0.84±0.04 | 0.88±0.06 | 0.97±0.04 | 0.99±0.10 | 0.92±0.09 | 0.96±0.04 | 0.94±0.05 | ||||||||
| * + | + | * | ||||||||||||||||
| Glucose | 0.96±0.11 | 0.85±0.19 | 0.53±0.17 | 0.78±0.10 | 1.05±0.22 | 0.94±0.10 | 0.93±0.10 | 0.85±0.12 | 0.88±0.09 | 0.94±0.03 | ||||||||
| % + | * | * # § $ | + § % | + # | ||||||||||||||
| Lactate | 0.91±0.04 | 0.90±0.08 | 0.75±0.15 | 0.85±0.09 | 0.82±0.07 | 0.99±0.06 | 0.95±0.01 | 0.95±0.03 | 0.94±0.04 | 0.95±0.02 | ||||||||
| * | + | + | * | |||||||||||||||
| Na+ | 0.97±0.02 | 0.95±0.01 | 0.97±0.01 | 0.96±0.01 | 0.95±0.00 | 0.97±0.03 | 0.94±0.02 | 0.95±0.01 | 0.95±0.01 | 0.95±0.01 | ||||||||
| * + | + § | * # | # § | * | * | |||||||||||||
| K+ | 0.89±0.10 | 0.88±0.09 | 0.77±0.12 | 0.88±0.07 | 0.86±0.06 | 1.00±0.02 | 0.89±0.03 | 0.91±0.04 | 0.94±0.03 | 0.90±0.10 | ||||||||
| + | + | |||||||||||||||||
| Ca++ | 0.79±0.14 | 0.82±0.17 | 0.81±0.12 | 0.90±0.09 | 0.91±0.03 | 1.03±0.04 | 0.79±0.23 | 0.88±0.18 | 0.91±0.04 | 0.96±0.06 | ||||||||
| * | * | |||||||||||||||||
SEM ± SD, P<0.05, pairs of symbols (+. #. *. §. $. %) indicate significant differences between the two groups. Creatinine values at baseline were significantly higher in the HES130 group compared with the other groups.
Sieving coefficients during haemofiltration
| . | Baseline (75/15) . | . | . | . | . | Qb/Qf max (200/40) . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SAL . | ALB . | HES200 . | HES130 . | GEL . | SAL . | ALB . | HES200 . | HES130 . | GEL . | ||||||||
| Creatinine | 0.66±0.14 | 0.57±0.17 | 0.5±0.17 | 0.37±0.13 | 0.61±0.16 | 0.78±0.13 | 0.80±0.24 | 0.97±0.40 | 0.49±0.13 | 0.81±0.22 | ||||||||
| + # | * | * + # | § | * | # | * + # § | + | |||||||||||
| Urea | 0.96±0.06 | 0.82±0.21 | 0.74±0.16 | 0.84±0.04 | 0.88±0.06 | 0.97±0.04 | 0.99±0.10 | 0.92±0.09 | 0.96±0.04 | 0.94±0.05 | ||||||||
| * + | + | * | ||||||||||||||||
| Glucose | 0.96±0.11 | 0.85±0.19 | 0.53±0.17 | 0.78±0.10 | 1.05±0.22 | 0.94±0.10 | 0.93±0.10 | 0.85±0.12 | 0.88±0.09 | 0.94±0.03 | ||||||||
| % + | * | * # § $ | + § % | + # | ||||||||||||||
| Lactate | 0.91±0.04 | 0.90±0.08 | 0.75±0.15 | 0.85±0.09 | 0.82±0.07 | 0.99±0.06 | 0.95±0.01 | 0.95±0.03 | 0.94±0.04 | 0.95±0.02 | ||||||||
| * | + | + | * | |||||||||||||||
| Na+ | 0.97±0.02 | 0.95±0.01 | 0.97±0.01 | 0.96±0.01 | 0.95±0.00 | 0.97±0.03 | 0.94±0.02 | 0.95±0.01 | 0.95±0.01 | 0.95±0.01 | ||||||||
| * + | + § | * # | # § | * | * | |||||||||||||
| K+ | 0.89±0.10 | 0.88±0.09 | 0.77±0.12 | 0.88±0.07 | 0.86±0.06 | 1.00±0.02 | 0.89±0.03 | 0.91±0.04 | 0.94±0.03 | 0.90±0.10 | ||||||||
| + | + | |||||||||||||||||
| Ca++ | 0.79±0.14 | 0.82±0.17 | 0.81±0.12 | 0.90±0.09 | 0.91±0.03 | 1.03±0.04 | 0.79±0.23 | 0.88±0.18 | 0.91±0.04 | 0.96±0.06 | ||||||||
| * | * | |||||||||||||||||
| . | Baseline (75/15) . | . | . | . | . | Qb/Qf max (200/40) . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SAL . | ALB . | HES200 . | HES130 . | GEL . | SAL . | ALB . | HES200 . | HES130 . | GEL . | ||||||||
| Creatinine | 0.66±0.14 | 0.57±0.17 | 0.5±0.17 | 0.37±0.13 | 0.61±0.16 | 0.78±0.13 | 0.80±0.24 | 0.97±0.40 | 0.49±0.13 | 0.81±0.22 | ||||||||
| + # | * | * + # | § | * | # | * + # § | + | |||||||||||
| Urea | 0.96±0.06 | 0.82±0.21 | 0.74±0.16 | 0.84±0.04 | 0.88±0.06 | 0.97±0.04 | 0.99±0.10 | 0.92±0.09 | 0.96±0.04 | 0.94±0.05 | ||||||||
| * + | + | * | ||||||||||||||||
| Glucose | 0.96±0.11 | 0.85±0.19 | 0.53±0.17 | 0.78±0.10 | 1.05±0.22 | 0.94±0.10 | 0.93±0.10 | 0.85±0.12 | 0.88±0.09 | 0.94±0.03 | ||||||||
| % + | * | * # § $ | + § % | + # | ||||||||||||||
| Lactate | 0.91±0.04 | 0.90±0.08 | 0.75±0.15 | 0.85±0.09 | 0.82±0.07 | 0.99±0.06 | 0.95±0.01 | 0.95±0.03 | 0.94±0.04 | 0.95±0.02 | ||||||||
| * | + | + | * | |||||||||||||||
| Na+ | 0.97±0.02 | 0.95±0.01 | 0.97±0.01 | 0.96±0.01 | 0.95±0.00 | 0.97±0.03 | 0.94±0.02 | 0.95±0.01 | 0.95±0.01 | 0.95±0.01 | ||||||||
| * + | + § | * # | # § | * | * | |||||||||||||
| K+ | 0.89±0.10 | 0.88±0.09 | 0.77±0.12 | 0.88±0.07 | 0.86±0.06 | 1.00±0.02 | 0.89±0.03 | 0.91±0.04 | 0.94±0.03 | 0.90±0.10 | ||||||||
| + | + | |||||||||||||||||
| Ca++ | 0.79±0.14 | 0.82±0.17 | 0.81±0.12 | 0.90±0.09 | 0.91±0.03 | 1.03±0.04 | 0.79±0.23 | 0.88±0.18 | 0.91±0.04 | 0.96±0.06 | ||||||||
| * | * | |||||||||||||||||
SEM ± SD, P<0.05, pairs of symbols (+. #. *. §. $. %) indicate significant differences between the two groups. Creatinine values at baseline were significantly higher in the HES130 group compared with the other groups.
Although TMP values were lowest in the HES130-treated groups, PF with HES130 supplementation caused sieving coefficients to increase from lower to higher flow rates. In contrast, GEL-based experiments led to decreasing sieving coefficients accompanied by increasing flow rates and TMP values. The latter indicated a strong limitation in maximal filter clearance from the side of sieving characteristics when PF and GEL infusion are combined.
The net filtration of albumin during low and high flow rates was lowest (P<0.05) in the HES130 and ALB groups. Thus, a loss of albumin due to plasmapheresis would be lowest in these two groups even though total protein clearance was similar to the other groups. A significant increase in the sieving coefficient for total protein was found only at high TMP values, which occurred in the HES200-group. Since these pressure values also led to spontaneous haemolysis, this ‘improvement’ in the sieving coefficient was not of clinical relevance. The results obtained between the groups for
During HF, there were significant differences between the study groups mainly at low flow rates. For example, both HES groups showed lower sieving coefficients with respect to most solutes. At higher flow rates, the still very low TMP values in the HES130 HF group were accompanied by a significantly lower sieving coefficient for creatinine when compared with the other groups showing higher TMP values. However, since the HES130 HF group provided the best relationship between TMP values and number of blocked capillaries, the clearance loss of ∼5–10% due to lower sieving coefficients would be counteracted by the option to run much higher flow rates (∼2-fold).
In both the HF and PF settings, low sieving coefficients were mainly accompanied by low TMP values, whereas even the highest TMP values did not lead to a deteriorated sieving profile during the experimental duration of 2 h.
Discussion
A number of different systems designed for extracorporeal detoxification are currently under evaluation in clinical studies. However, although early results relating to application in acute-to-chronic liver failure may have been promising, there is not yet sufficient evidence to recommend its routine use [11]. To address the important issue of therapy using high clearance features in intensive care medicine, we proposed a different hypothesis to identify strategies for increasing filter clearance [7,8]. One concept resulting from this hypothesis was to utilize the capacities of certain colloids to reduce blood cell and plasma protein agglomeration, which was assessed in the present study.
To obtain maximal filter clearance during PF and HF, flow rates and sieving coefficients are of relevance; however, flow rates are still a major problem for filter clearance of high quality membranes. Because haemocompatibility is related to TMP values, the most important criteria for beneficial effects from a volume substitute are low TMP values even during high flow rates and minimized haemolysis. Here we found that the study groups with HES130 and ALB supplementation demonstrated the best results (e.g., low TMP values or stable values of fHb enabled high flow rates with constant or even increasing sieving coefficients). In contrast, GEL supplementation led to decreasing sieving coefficients that were already visible during 2 h of in vitro haemofiltration and to high numbers of blocked hollow fibres. These findings clearly indicate that this substance represents a strong limitation for filter clearance. Unfortunately, statistical analyses revealed sporadically slight differences in blood composition at baseline. However, even if significant, these differences were opposed by overall wide ranges in baseline values. Furthermore, these differences were inconsistent between the groups with respect to their impact on blood rheology. Thus, neither volume substitute was favoured.
The ranking of ALB and HES130 as volume substitutes of first choice may be questioned from a clinical point of view. ALB was banned 2 years ago for use in volume management in intensive care medicine by certain clinicians since mortality rate was reportedly higher compared with other solutions [12]. However, recent studies reported that ALB does not increase mortality rate. Thus, cost : benefit ratio is the last remaining point of discussion [13]. HES preparations are thought to increase the risk of renal failure. For example, an important publication [14] indicated that HES200/0.62, a preparation not investigated in the present study, was an independent risk factor for renal failure. However, the mortality rate or days in intensive care were not higher in the HES200/0.62-treated group, and mechanical ventilation was found to be the main factor for increase in risk of renal failure. In addition, this study has been widely criticized due to limitations in setting and documentation [see published comments in 14,15]. Interestingly, recent recommendations favouring GEL over HES or ALB preparations have lacked considerations of adverse effects arsing from GEL preparations (e.g., anaphylactic reactions, increased cell aggregability impairing rheology, coagulation compromising effects) [16]. Furthermore, there has been no evidence from clinical studies to support a ban on all HES preparations and ALB from treatment strategies in intensive care medicine independent of age and underlying diseases [13–17]. Finally, all of these preparations may be administered during surgery or haemorrhagic shock prior to the need of patients for extracorporeal treatment [17]. Thus, the complexity of this topic has led to inconsistent and rapidly changing viewpoints and ‘state-of-the-art’ knowledge of volume management remains in conflict. Because of this, there is still high demand for information obtained from standardized experimental studies.
However, interactions between a filtration device itself and the volume substitute used for basic treatment have not yet been investigated or even discussed in the clinical setting. In addition, the ranking of volume substitutes that takes into account the overall performance of two single results may be of special interest. Even when haemolysis was significant, supplementation with GEL caused suitable reductions in fHb increases to very low levels. This is in accordance with the findings of Sümpelmann et al. [18] who described GEL preparations that were protective against mechanical stress for erythrocytes during extracorporeal circulation. Nevertheless, in cases of PF and HF, this phenomenon was accompanied by an increased cell and plasma agglomeration, and decreasing sieving coefficients during PF which led to deterioration of the overall performance of the filtration procedure. To summarize, the protective effect of a GEL preparation on erythrocytes [15] may not apply to filtration procedures using hollow fibre membranes.
Interestingly, the HES130 HF group showed increasing fibrinogen concentrations from baseline to final values. In recirculating in vitro HF, systematic increases in high molecular weight plasma solutes are method based because removal of ultrafiltrate during filtrate sampling for analyses leads to a reduction in plasma water. However, in the HES130-HF group, fibrinogen concentrations exceeded the final values expected from a method-based procedure. Fibrinogen concentrations were measured using functional plasma coagulation by correlating coagulation time with fibrinogen concentrations. Because our system did not include fibrinogen synthesizing cells, genuine increases in fibrinogen concentrations were not possible. Thus, we assume an interaction between HES130 molecules and fibrin formation reduced the coagulation time, which in turn falsely indicated an increased fibrinogen concentration. This conclusion is in accordance with the microscopy findings that ‘fibrin molecules’ found in the HES130 PF group capillaries were of another size and shape than those found in other groups. However, our laboratory results are strongly supported by Strauss et al. [19]. They showed that HES may lead to a faster transformation of fibrinogen to fibrin. Thus, our observations may indicate a fibrinogen-saving effect of HES130. In general, differences found in our study between HES200 and HES130 are in accordance with a clinical study which demonstrated that HES130 had fewer adverse effects on coagulation during major orthopaedic surgery [20]. If our current results are confirmed in in vivo studies, our findings for HES200 preparations will likely contribute to the list of risk factors for this HES type, whereas results for HES130 may contribute to the list of ‘pro’-aspects. Taken together, these findings justify a re-examination of research on ALB and HES130 as adjunct to anticoagulation in patients who are suffering from severe hypovolaemia and are at high risk for bleeding during extracorporeal detoxification.
Our results showing interactions between volume substitutes and coagulation are in accordance with various clinical studies. Nonetheless, the need to accelerate processes of deterioration during whole blood in vitro settings has led to differences in clinical outcomes (e.g., upper levels of Hct and protein that are seen under clinical circumstances and acceptance of high pressure levels). Thus, further efforts for a reliable extrapolation from in vitro results to the in vivo situation are necessary. In particular, there is a lack of studies that clearly distinguish between the impact of colloids on coagulation via influences on cell aggregation versus a direct molecular interaction with coagulation factors. This limits comparisons and discussion of current study results. Nevertheless, the effects on blood cell aggregability discussed in the present study may provide a new tool for low dose anticoagulation.
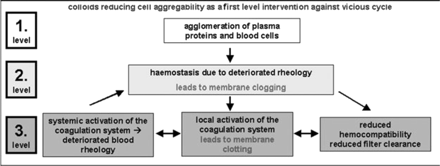
To summarize, it now appears of importance to intensify research on the use of different rheological features of colloidal volume substitutes to control the vicious cycle of blocked capillary membranes in hollow fibre modules for ultra- and plasma-filtration. Colloids may provide a means for intervention at the first level of initiation of the vicious cycle of blocked capillaries, increasing pressure profiles, membrane fouling, and activation of the coagulation system (Figure 4). The prevention of adverse side effects of extracorporeal detoxification by preventing haemostasis as a first level intervention would be of great advantage compared to the standard third level intervention routinely used via different anticoagulation regimes, which still lead to life threatening complications.
Principle of the vicious cycle of deteriorated blood rheology and haemocompatibility. First and second levels could be counteracted by colloids reducing cell and plasma protein agglomeration; thrid level requires increasing anticoagulation.
This study was supported by ‘START-RWTH Aachen’, Gambro Dialysatoren, Hechingen; Fresenius Kabi Deutschland GmbH, Bad Homburg and Else Kröner-Fresenius Foundation. Furthermore, we cordially thank our laboratory assistant, Renate Nadenau, and our students Wolf Siepen, Britta Portz and Andrea Heuer for their committed and professional support throughout this study.
Conflict of interest statement. None declared.
References
Joannes-Boyau O, Rapaport S, Bazin R, Fleureau C, Janvier G. Impact of high volume hemofiltration on hemodynamic disturbance and outcome during septic shock.
Clemmesen JO, Kondrup J, Nielsen LB, Larsen FS, Ott P. Effects of high-volume plasmapheresis on ammonia, urea, and amino acids in patients with acute liver failure.
Hughes RD. Review of methods to remove protein-bound substances in liver failure.
Swartz R, Pasko D, O'Toole J, Starmann B. Improving the delivery of continuous renal replacement therapy using citrate anticoagulation.
de Pont AC, Oudemans-van Straaten HM, Roozendaal KJ, Zandstra DF. Nadroparin versus dalteparin anticoagulation in high volume, continuous venovenous hemofiltration: a double-blind, randomized, crossover study.
Unger JK, Horn NA, Kashefi A, Blumberg A, Klosterhalfen B, Rossaint R. The influence of hypoalbuminemia on maximal flow rates and transmembrane pressure during plasmapheresis—an in vitro study.
Unger JK, Janssen VR, Kashefi A et al. Enhancing filtration rates by the use of blood flow around the capillaries of plasmafilters—an in vitro study.
Lacombe C, Bucherer C, Ladjouzi J, Lelievre JC. Competitive role between fibrinogen and albumin on thixotropy of red cell suspensions.
Maeda N, Shiga T. Opposite effect of albumin on the erythrocyte aggregation induced by immunoglobulin G and fibrinogen.
Liu JP, Gluud LL, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for liver failure.
Bunn F, Alderson P, Hawkins V. Colloid solutions for fluid resuscitation.
Alderson P, Bunn F, Lefebvre C, Li Wan Po A, Roberts I, Schierhout G. Human albumin solution for resuscitation and volume expansion in critically ill patients.
Schortgen F, Lacherade J-C, Bruneel F et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study.
Boldt J, Priebe H-J. Intravascular volume replacement therapy with synthetic colloids: is there an influence on renal function?
Ragaller MJR, Theilen H, Koch T. Volume replacement in critically ill patients with acute renal failure.
Dehne MG, Mühling J, Sablotzki A, Dehne KL, Sucke N, Hempelmann G. Hydroxyethyl Starch (HES) does not directly affect renal function in patients with no prior renal impairment.
Sümpelmann R, Schürholz T, Marx G, Zander R. Protective effects of plasma replacement fluids on erythrocytes exposed to mechanical stress.
Strauss RG, Stump DC, Henriksen RA, Saunders R. Effects of hydroxyethyl starch on fibrinogen, fibrin clot formation, and fibrinolysis.
Author notes
1Department of Anaesthesiology and 2Department of Clinical Chemistry, University Hospital Aachen, Rheinisch-Westfälische Technische Hochschule Aachen, Aachen, Germany, 3Department of Comparative Medicine and Experimental Animal Sciences and 4Department of Paediatric Pneumology and Immunology, Charité, Campus Virchow, Humboldt-University, Berlin, Germany





Comments