-
PDF
- Split View
-
Views
-
Cite
Cite
Wei Zhou, Amanda L Anderson, Adrian P Turner, Geoffry N De Iuliis, Adam McCluskey, Eileen A McLaughlin, Brett Nixon, Characterization of a novel role for the dynamin mechanoenzymes in the regulation of human sperm acrosomal exocytosis, Molecular Human Reproduction, Volume 23, Issue 10, October 2017, Pages 657–673, https://doi.org/10.1093/molehr/gax044
Close - Share Icon Share
Abstract
Does dynamin regulate human sperm acrosomal exocytosis?
Our studies of dynamin localization and function have implicated this family of mechanoenzymes in the regulation of progesterone-induced acrosomal exocytosis in human spermatozoa.
Completion of an acrosome reaction is a prerequisite for successful fertilization in all studied mammalian species. It follows that failure to complete this unique exocytotic event represents a common aetiology in the defective spermatozoa of male infertility patients that have failed IVF in a clinical setting. Recent studies have implicated the dynamin family of mechanoenzymes as important regulators of the acrosome reaction in murine spermatozoa. The biological basis of this activity appears to rest with the ability of dynamin to polymerize around newly formed membrane vesicles and subsequently regulate the rate of fusion pore expansion. To date, however, the dynamin family of GTPases have not been studied in the spermatozoa of non-rodent species. Here, we have sought to examine the presence and functional significance of dynamin in human spermatozoa.
Dynamin expression was characterized in the testis and spermatozoa of several healthy normozoospermic individuals. In addition, we assessed the influence of selective dynamin inhibition on the competence of human spermatozoa to undergo a progesterone-induced acrosome reaction. A minimum of five biological and technical replicates were performed to investigate both inter- and intra-donor variability in dynamin expression and establish statistical significance in terms of the impact of dynamin inhibition.
The expression and the localization of dynamin in the human testis, epididymis and mature spermatozoa were determined through the application of immunofluorescence, immunoblotting and/or electron microscopy. Human semen samples were fractionated via density gradient centrifugation and the resultant populations of good and poor quality spermatozoa were induced to capacitate and acrosome react in the presence or absence of selective dynamin inhibitors. The acrosome integrity of live spermatozoa was subsequently assessed via the use of fluorescently conjugated Arachis hypogea lectin (PNA). The influence of dynamin phosphorylation and the regulatory kinase(s) responsible for this modification in human spermatozoa were also assessed via the use of in situ proximity ligation assays and pharmacological inhibition. In all experiments, ≥100 spermatozoa were assessed/treatment group and all graphical data are presented as the mean values ± SEM, with statistical significance being determined by ANOVA.
Dynamin 1 (DNM1) and DNM2, but not DNM3, were specifically localized to the acrosomal region of the head of human spermatozoa, an ideal position from which to regulate acrosomal exocytosis. In keeping with this notion, pharmacological inhibition of DNM1 and DNM2 was able to significantly suppress the rates of acrosomal exocytosis stimulated by progesterone. Furthermore, our comparison of dynamin expression in good and poor quality spermatozoa recovered from the same ejaculate, revealed a significant reduction in the amount of DNM2 in the latter subpopulation of cells. In contrast, DNM1 was detected at equivalent levels in both subpopulations of spermatozoa. Such findings are of potential significance given that the poor quality spermatozoa proved refractory to the induction of a progesterone stimulated acrosome reaction. In seeking to identify the regulatory influence of progesterone on DNM2 function, we were able to establish that the protein is a substrate for CDK1-dependent phosphorylation. The functional significance of DNM2 phosphorylation was illustrated by the fact that pharmacological inhibition of CDK1 elicited a concomitant suppression of both DNM2-Ser764 phosphorylation and the overall rates of progesterone-induced acrosomal exocytosis.
N/A.
This was an in vitro study performed mainly on ejaculated human spermatozoa. This experimental paradigm necessarily eliminates the physiological contributions of the female reproductive tract that would normally support capacitation and acrosomal responsiveness.
This study identifies a novel causative link between dynamin activity and the ability of human spermatozoa to complete a progesterone-induced acrosome reaction. Such findings encourage a more detailed analysis of the contribution of dynamin dysregulation as an underlying aetiology in infertile males whose spermatozoa are unable to penetrate the zona pellucida.
This research was supported by a National Health and Medical Research Council of Australia Project Grant (APP1103176) awarded to B.N. and E.A.M. The authors report no conflict of interest.
Introduction
In all mammalian species that have been studied, completion of an acrosome reaction is accepted as a prerequisite for successful fertilization in vivo (Florman et al., 2004; Avella and Dean, 2011). This unique exocytotic process is initiated by multiple fusion events between the outer acrosomal membrane and the overlying plasma membrane (Barros et al., 1967). As this wave of vesiculation spreads across the anterior region of the sperm head, it facilitates the destablization of the acrosomal structure, formation of hybrid membrane vesicles and the concomitant release of acrosomal contents (Barros et al., 1967). This, in turn, enables sperm penetration of the resilient zona pellucida matrix, and provides the cell with access to the oocyte plasma membrane. Recent work has drawn into question the long held view that the acrosome reaction proceeds in an ‘all or none’ manner, with accumulating evidence suggesting that the process may instead comprise several intermediate phases, each with their own set of functional consequences (Buffone et al., 2012, 2014). Irrespective of this, several features of acrosomal exocytosis, including the relatively slow kinetics at which it proceeds, underscore the highly specialized nature of this secretory process and the prospect that is governed by complex molecular mechanisms (Belmonte et al., 2016).
In keeping with this notion, the capacity to complete a physiological acrosome reaction necessitates that spermatozoa have first completed a process of functional maturation, known as capacitation, during their ascent of the female reproductive tract (Stival et al., 2016). Among the many biochemical and biophysical changes that accompany capacitation, the sperm membrane becomes increasingly fusogenic, owing to alterations in its lipid architecture and the release of decapacitation factors (Nixon et al., 2006; Aitken and Nixon, 2013). These combined events serve to modulate intracellular ion concentrations leading to hyperpolarization of the sperm plasma membrane and triggering complex signalling cascades that culminate in increased protein phosphorylation (Visconti et al., 2011). While these processes prime the cell for completion of an acrosome reaction, the precise physiological stimulus responsible for the induction of this exocytotic event remains a matter of some controversy (Buffone et al., 2014). Indeed, the long held view that zona pellucida ligands act as the key stimulus for induction of acrosomal exocytosis have recently been drawn into question (Baibakov et al., 2007) in favour of alternative models, which suggest that the timing of the acrosomal exocytosis may actually precede that of zona adhesion (Inoue et al., 2011). Such timing may account for the ability of progesterone, a signalling molecule that sperm encounter within the oviduct prior to zona adhesion, to elicit the induction of acrosomal exocytosis in various species, including the human (Meyers et al., 1995; Meizel et al., 1997; Thérien and Manjunath, 2003). Progesterone, in turn, reportedly acts via its ability to modulate an increase in cytosolic Ca2+ levels (Blackmore et al., 1990).
The impetus for further investigation of the regulation of acrosomal exocytosis in human spermatozoa stems, at least in part, from the recognition that a failure to complete this event represents a relatively common aetiology associated with the defective spermatozoa of male infertility patients. Indeed, diagnosis of sperm dysfunction suggests that such a defect may account for almost one-third of all cases of failed IVF in couples seeking recourse to assisted reproductive programs due to male factor infertility (Muller, 2000; Liu and Baker, 2003). Notably, these patients commonly present with otherwise normal semen characteristics, suggesting that the defect may be associated with the molecular machinery responsible for the control of acrosomal exocytosis rather than overt morphological defects in their spermatozoa. While such machinery is undoubtedly complex, our recent work in the mouse model has raised the prospect that the dynamin family of mechanoenzymes may hold a critical role in the regulation of the acrosome reaction (Reid et al., 2012; 2015).
The dynamin family of proteins comprise a group of large GTPases that have been extensively researched in the context of their ability to manipulate membrane vesicles during the combined processes of endo- and exo-cytosis (Shpetner and Vallee, 1989; Williams and Kim, 2014; Jackson et al., 2015). Such functions are largely attributed to the ability of dynamin to self-oligomerize into cylindrical helices around the neck of nascent vesicles (Antonny et al., 2016). In one of the most widely accepted models of dynamin action, GTP hydrolysis subsequently drives conformational changes that lead to constriction of both the polymer and the membrane beneath, thus promoting vesicle scission and release from the parent membrane (Morlot and Roux, 2013). It has also been shown that dynamin has the ability to regulate the rate of fusion pore expansion between the membranes and newly formed vesicles, thus controlling the amount of cargo released from the vesicles (Anantharam et al., 2011; Jackson et al., 2015). Such a model is in keeping with the protracted timeframe, and progressive release, of the acrosomal contents that have been documented during acrosomal exocytosis in mammalian spermatozoa (Buffone et al., 2014; Belmonte et al., 2016).
The existence of three canonical dynamin members (DNM1, DNM2 and DNM3), as well as several dynamin-like proteins, has been recorded in mammalian species (Antonny et al., 2016). Being encoded by three different genes, DNM1, DNM2 and DNM3 are characterized by differential patterns of expression within distinct tissues of the body. In this context, DNM1 is predominantly found in the central nervous system (Ferguson et al., 2007), DNM2 is ubiquitously expressed throughout the body (Cook et al., 1994) and DNM3 appears to reside mainly in the brain and testis (Cao et al., 1998). Studies conducted in our own, and independent laboratories, have recently begun to explore the expression and functional significance of dynamin in the male reproductive tract of murine models (Iguchi et al., 2002; Lie et al., 2006; Kusumi et al., 2007; Vaid et al., 2007; Zhao et al., 2007; Reid et al., 2012; 2015; Redgrove et al., 2016; Zhou et al., 2017). Thus, prominent expression of DNM2 has been documented in the mouse testes where the protein appears to localize to developing germ cells (Redgrove et al., 2016). Accordingly, conditional ablation of the Dnm2 gene leads to the complete arrest of spermatogenesis and a corresponding phenotype of male infertility (Redgrove et al., 2016). DNM1 has been identified in mouse germ cells where it appears to be restricted to the developing acrosome of round spermatids (Reid et al., 2012). However, unlike Dnm2, the targeted ablation of Dnm1 did not precipitate overt changes in germ cell development or male infertility (Redgrove et al., 2016). In contrast, DNM3 expression within the testis appears to have minimal overlap with the germ cell population (Vaid et al., 2007; Reid et al., 2012). Importantly in the context of this study, we have also shown that both DNM1 and DNM2, but not DNM3, are retained in mature mouse spermatozoa where they co-localize within the peri-acrosomal region of the sperm head (Reid et al., 2012). From this position, DNM1 and DNM2 appear to exert an important regulatory influence over acrosomal exocytosis, such that their selective pharmacological inhibition significantly compromises the ability of mouse spermatozoa to complete a progesterone-induced acrosome reaction (Reid et al., 2012). In agreement with such findings, independent research has shown the DNM2 also forms productive interactions with complexin I (Zhao et al., 2007), an important element of the SNARE family of proteins that themselves have been implicated in regulation of acrosomal exocytosis (Buffone et al., 2014). Accordingly, this interaction has been mapped to the acrosomal region of mouse spermatozoa (Zhao et al., 2007).
Notwithstanding promising data generated thus far in rodent models, to the best of our knowledge, there is presently no research directly exploring the presence and/or functional significance of dynamin isoforms in human spermatozoa. Thus, the aim of this study was to characterize the expression pattern of the canonical dynamin family members (DNM1, DNM2 and DNM3) in the human testis and ejaculated spermatozoa, as well as assess their potential role in the regulation of acrosomal exocytosis in our own species.
Materials and Methods
Ethics statement
The experiments described in this study were conducted with human semen samples obtained with informed written consent from a panel of healthy normozoospermic donors (University student volunteers) assembled for the Reproductive Science Group at the University of Newcastle. All experiments were performed in accordance with protocols approved by the University of Newcastle Human Research and Ethics Committee.
Reagents
Unless specified, chemical regents were purchased from Sigma-Aldrich (St. Louis, MO, USA) and were of molecular biology or research grade. Rabbit polyclonal antibody against dynamin 1 (ab108458) was purchased from Abcam (Cambridge, England, UK); goat polyclonal antibody against dynamin 2 (sc-6400) and IZUMO1 (sc-79 543), mouse monoclonal antibody against CDC2 (CDK1) (sc-54) and rabbit polyclonal antibody against CDK5 (sc-173) were from Santa Cruz Biotechnology (Dallas, TX, USA); rabbit polyclonal anti-dynamin 2 (PA5-19 800) and sheep polyclonal anti-phospho-dynamin 1 (Ser-778) antibodies were from Thermo Fisher Scientific (Eugene, OR, USA); rabbit polyclonal antibody against dynamin 3 (14 737-1-AP) was purchased from Proteintech Group (Chicago, IL, USA); mouse monoclonal antibody against α-tubulin (T5168) was from Sigma-Aldrich. Alexa Fluor 594-conjugated goat anti-rabbit, donkey anti-goat and goat anti-mouse, Alexa Fluor 488-conjugated donkey anti-sheep and goat anti-rabbit were all purchased from Thermo Fisher Scientific. Anti-rabbit immunoglobulin G (IgG) conjugated to horse radish peroxidase (HRP) was supplied by Millipore (Chicago, IL, USA) and anti-sheep IgG-HRP was supplied by Abcam. Full details of the primary and secondary antibodies are also provided in Supplementary Table S1. Bovine serum albumin (BSA) was purchased from Research Organics (Cleveland, OH, USA). d-glucose was supplied by Ajax Finechem (Auburn, NSW, Australia). Percoll, HEPES and nitrocellulose were from GE healthcare (Buckinghamshire, England, UK). Precast 4–20% polyacrylamide gels were purchased from Bio-Rad Laboratories (Gladesville, NSW, Australia). Minicomplete protease inhibitor cocktail tablets were supplied by Roche (Sandhoferstrasse, Mannheim, Germany). Mowiol 4–88 was from Calbiochem (La Jolla, CA, USA), paraformaldehyde (PFA) was obtained from ProSciTech (Thuringowa, QLD, Australia). Human testis lysate was purchased from Santa Cruz Biotechnology. CDK1 inhibitor (217 695) was from Millipore. Dynamin inhibitors, Dynasore and Dyngo 4a were purchased from Tocris Bioscience (Bristol, England, UK) and Abcam, respectively. Dyngo-⊖ (an inactive chemical analogue of both the Dynasore and Dyngo 4a inhibitors) was generated in our laboratory as previously described (McCluskey et al., 2013).
Human semen analysis
All semen samples used in this study were assessed according a specialized methodological checklist (Björndahl et al., 2015). Briefly, semen samples were collected following sexual abstinence of at least 2 days. After collection, all samples were kept at 37°C and were de-identified prior to delivery to the laboratory except for assignment of a unique identification number. Sample analysis was initiated after completion of liquefaction and within 1 h of ejaculation. At least 100 cells were assessed in each of two duplicates for determination of cell motility, viability and morphology, with at least five microscope fields of view being examined in each duplicate count. Sperm morphology was assessed in accordance with WHO criteria (World Health Organization, 2010) in the absence of papanicolaou, Shorr or Diff-Quik staining using ×400 magnification and bright field microscopy (Olympus CX40, Olympus, Sydney, Australia). Motility assessment was performed using phase contrast microscope optics (×400 magnification), with cells being classified as either motile (i.e. sperm that displayed any form of motility ranging from rapid progressive to non-progressive) or immotile. The aliquot of semen used for assessment of sperm concentration was sampled using a positive displacement pipette with care taken to avoid the introduction of bubbles during aspiration. No assessment of anti-sperm antibodies was performed, however, all samples were free of abnormal clumping (aggregates and agglutinates) and the presence of excessive inflammatory cells (<1 million/ml). No biochemical markers for prostatic, seminal vesicle or epididymal secretions were analysed owing to our focus on spermatozoa.
Human spermatozoa preparation and capacitation
Human semen samples were fractionated over a discontinuous Percoll density gradient (comprising 40 and 80% Percoll suspensions) by centrifugation at 500 × g for 30 min (Redgrove et al., 2012). After centrifugation, two fractions comprising the interface of the 40 and 80% Percoll suspensions and the pellet at the base of the 80% Percoll suspension were collected individually prior to being resuspended in a non-capacitating (non-cap) formulation of Biggers, Whitten and Whittingham medium (BWW) (Biggers et al., 1971) containing 1 mg/ml polyvinyl alcohol (PVA) but prepared without HCO3−(osmolarity of 290–310 mOsm/kg) (Bromfield et al., 2015a). A subset of these cells were assessed for motility and concentration while the remainder were washed by centrifugation at 500 × g for 15 min. The sperm cells collected from the base of the 80% Percoll suspension are nominally referred as ‘good quality’ or ‘mature’ spermatozoa, whereas those partitioning at the 40/80% Percoll interface are referred to as ‘poor quality’ spermatozoa (Aitken et al., 1993). Unless otherwise stated, good quality spermatozoa were used throughout our experiments following their resuspension in medium appropriate to experimental requirements.
To induce capacitation in vitro, spermatozoa were incubated in a formulation of BWW (91.5 mM NaCl, 4.6 mM KCl, 1.7 mM CaCl22 H2O, 1.2 mM KH2PO4, 1.2 mM MgSO47 H2O, 25 mM NaHCO3, 5.6 mM D-glucose, 0.27 mM sodium pyruvate, 44 mM sodium lactate, 5 U/ml penicillin, 5 mg/ml streptomycin, 20 mM HEPES buffer, 1 mg/ml PVA (substituted for BSA; osmolarity of 290–310 mOsm/kg), 3 mM pentoxifylline and 5 mM dibutyryl cyclic adenosine monophosphate) that has previously been optimized for the induction of human sperm capacitation as assessed via tyrosine phosphorylation status, zona pellucida-binding competence and, importantly in the context of this study, the ability to complete an acrosome reaction (Mitchell et al., 2007). Where indicated, the capacitating medium was supplemented with either dimethyl sulfoxide (DMSO) (vehicle control), Dynasore, Dyngo 4a or Dyngo-⊖. Spermatozoa were incubated at a concentration of 10 × 106 cells/ml at 37°C under an atmosphere of 5% CO2:95% air for 3 h with a gentle inversion every 30 min to prevent settling of the cells (Mitchell et al., 2007; 2008; Redgrove et al., 2011). After incubation, spermatozoa were prepared for assessment of their capacity to undergo an acrosomal reaction utilizing the assay protocols outlined below.
Acrosomal reaction assays
Following appropriate incubation, spermatozoa (non-capacitated, capacitated, dynamin inhibited or Dyngo-⊖ treated) were induced to acrosome react by supplementation of media with either 2.5 μM A23187 for 30 min or 15 μM progesterone (pH 7.00–7.05) for 2 h in the presence of dynamin inhibitors or vehicle controls prepared at the same concentration as utilized during the initial capacitation incubation. The spontaneous rates of acrosome loss were assessed via the inclusion of a capacitated sperm control group (designated Cap control), which were prepared under identical incubation conditions with the exception that they did not receive an A23187 or progesterone stimulus. At the completion of this induction period, sperm motility and viability were assessed (Bromfield et al., 2015a) to ensure neither parameter was compromised by any of the treatments. The cells were then incubated in pre-warmed hypo-osmotic swelling media (HOS; 0.07% w/v sodium citrate; 1.3% w/v fructose) for another 30 min at 37°C. After being fixed in 4% PFA, spermatozoa were aliquoted onto 12-well slides, air-dried and permeabilized with ice cold methanol for 10 min. Cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated PNA (1 μg/μl) at 37°C for 15 min, and the acrosomal status of viable cells (possessing coiled tails as a result of incubation in HOS medium) were verified using fluorescence microscopy (Zeiss Axio Imager A1, Jena, Thuringia, Germany). The wavelengths of the microscopic filters used for excitation and emission were 474 and ~527 nm.
Immunofluorescent localization
Human testis and epididymis sections used in this study were kindly provided by Dr Zhuo Yu of Shanghai Jiaotong University. These tissues were collected from donors diagnosed with prostatic carcinoma but showing normal morphology of both the testis and epididymis. Tissues were fixed in fresh Bouin's solution, embedded in paraffin and sectioned at 5 μm thickness. Embedded tissue was dewaxed, rehydrated and then subjected to antigen retrieval as previously reported (Zhou et al., 2017). After being blocked with 3% BSA/phosphate-buffered saline (PBS) at 37°C for 1 h, slides were incubated with primary antibodies at 4°C overnight (for specific dilution rates of all antibodies see Supplementary Table S1). After three washes in PBS, slides were incubated with appropriate Alexa Fluor conjugated secondary antibodies at 37°C for 1 h. Following additional washes, slides were incubated with PNA at 37°C for 15 min and counterstained with nuclear dyes; propidium iodide (5 μg/ml) or 4′,6-diamidino-2-phenylindole (2 μg/ml). Slides were then washed and mounted with a 10% Mowiol 4–88 with 30% glycerol in 0.2 M Tris (pH 8.5) and 2.5% 1, 4-diazabicyclo-(2.2.2)-octane (DABCO). Staining patterns were recorded using fluorescence microscopy (Zeiss Axio Imager A1). The wavelengths of the microscopic filters used for excitation and emission were 474 nm and ~527 nm (Alexa Fluor 488 and propidium iodide), and 585 nm and ~615 nm (Alexa Fluor 594). Alternatively, confocal microscopy (Olympus IX81) was used for detection of fluorescent-labelling patterns using excitation and emission filters of wavelength 473 nm and 485–545 nm (Alexa Fluor 488), and 559 nm and 570–670 nm (propidium iodide).
For immunofluorescent staining of human spermatozoa, cells were fixed in 4% (w/v) PFA for 15 min at room temperature, washed in 0.05 M glycine/PBS and settled onto poly-L-lysine treated coverslips at 4°C overnight. For detection of the CDK1 kinase, cells were then subjected to antigen retrieval via immersion in 100 mM Tris, 5% urea (pH 9.5) at 90°C for 10 min (Reid et al., 2015) and permeabilized with 0.1% Triton X-100 for 10 min. For detection of all other proteins, spermatozoa were permeabilized with ice cold methanol for 10 min (no antigen retrieval required). Following PBS washes, cells were blocked with 3% BSA/PBS and immunolabelled as described for human testis and epididymis slides.
Electron microscopy
Human spermatozoa were fixed in 4% (w/v) PFA containing 0.5% (v/v) glutaraldehyde. Cells were then processed via dehydration, infiltration and embedding in LR White resin. Sections (80 nm) were cut with a diamond knife (Diatome Ltd, Bienne, Switzerland) on an EM UC6 ultramicrotome (Leica Microsystems, Vienna, Austria) and placed on 200-mesh nickel grids. Subsequent washes were performed using PBS containing 1% foetal calf serum (FCS). After being blocked with 10% FCS + 1% cold water fish gelatin in PBS (30 min), sections were incubated with primary antibodies overnight at 4°C. An appropriate secondary antibody conjugated to 10 nm (anti-goat) gold particles was incubated on grids for 90 min at room temperature. Sections were counterstained in 2% (w/v) uranyl acetate. Micrographs were taken on a Tecnai 12 transmission electron microscope (FEI Company) at 120 kV.
SDS-PAGE and immunoblotting
Protein was extracted from human spermatozoa via boiling in SDS extraction buffer (0.375 M Tris pH 6.8, 2% w/v SDS, 10% w/v sucrose, protease inhibitor cocktail) at 100°C for 5 min. Insoluble material was removed by centrifugation (17 000 × g, 10 min, 4°C) and soluble protein remaining in the supernatant was quantified using a BCA protein assay kit (Thermo Fisher Scientific). Extracellular vesicles were isolated from seminal plasma using an optimized OptiPrep density gradient protocol (Reilly et al., 2016) and proteins were extracted and quantified as described for human sperm samples. Equivalent amounts of protein (10 μg) were boiled in SDS-PAGE sample buffer (2% v/v mercaptoethanol, 2% w/v SDS, and 10% w/v sucrose in 0.375 M Tris, pH 6.8, with bromophenol blue) at 100°C for 5 min, prior to be resolved by SDS-PAGE (150 V, 1 h) and transferred to nitrocellulose membranes (350 mA, 1 h). Similar protocols were also used in order to prepare human testis protein (sc-363 781) for immunoblotting. Membranes were then blocked and incubated with appropriate antibodies raised against target proteins by using optimized conditions as previous described (Zhou et al., 2017). Briefly, blots were washed three times × 10 min with either PBS supplemented with 0.5% (v/v) Tween-20 (PBST) (dynamin 1), or Tris-buffered saline with 0.1% (v/v) Tween-20 (TBST) (dynamin 2, dynamin 3, dynamin pSer778 and α-tubulin), before being probed with appropriate HRP-conjugated secondary antibodies (see Supplementary Table S1). After three additional further washes, labelled proteins were detected using an enhanced chemiluminescence kit (GE Healthcare). Where appropriate labelled protein band intensity was determined by densitometry using Image J-win64 software (Gassmann et al., 2009; Xia et al., 2016).
Immunoprecipitation
Capacitated spermatozoa were progesterone treated for 1 h. After treatment, cell lysis was performed on a total population of ~100 × 106 cells in 200 μl cell lysis buffer (10 mM CHAPS, 10 mM HEPES, 137 mM NaCl and 10% glycerol supplemented with a protease inhibitor cocktail) at 4°C for 2 h. Following centrifugation (15 000 × g, 4°C for 10 min), the supernatant containing soluble protein was transferred to a clean tube and precleared by incubation with 50 μl aliquots of a protein G Dynabead slurry at 4°C for 30 min. A total of 3 μg of anti-DNM2 antibody was then added to the precleared cell lysate followed by overnight incubation at 4°C with constant rotation. At the completion of this incubation period, protein G Dynabeads were added and the suspension returned to rotation for a further 30 min at 4°C to facilitate the precipitation of target antigens. The beads were subsequently washed three times in PBS, prior to resuspension in SDS loading buffer and boiling for 5 min to elute target proteins. These proteins were then subjected to immunoblotting with either anti-DNM2 or anti-DNM1 serine-778 antibodies.
Duolink proximity ligation assay
Human spermatozoa were prepared for Duolink in situ proximity ligation assays (PLAs) as previously described. In brief, capacitated populations of human spermatozoa were incubated in the presence or absence of progesterone (15 μM) for 1 h. Non-capacitated cells were incubated for the same time period in non-cap BWW medium alone. Following incubation, sperm motility and viability were recorded and the cells were then fixed, washed and settled onto poly-l-lysine treated coverslips at 4°C overnight. After antigen retrieval and permeabilization (see immunofluorescence; all antibodies used for PLA were first assessed via immunofluorescence for compatibility of antigen retrieval conditions), PLA labelling was conducted according to the manufacturers’ instructions (OLINK Biosciences, Uppsala, Sweden) using pairs of primary antibodies comprising anti-CDK1 and anti-DNM2, anti-CDK1 and anti-DNM1 or anti-CDK1 and anti-IZUMO1 (irrelevant antibody control). Appropriate synthetic oligonucleotide-conjugated secondary antibodies (OLINK Biosciences) were subsequently applied for incubation at 37°C 1 h. After ligation and amplification, sperm cells were viewed using fluorescence microscopy (Zeiss Axio Imager A1). In this assay, target proteins residing within a maximum distance of 40 nm from each other are detected via the generation of foci of red fluorescent dots. A threshold of three or more fluorescent foci within the sperm head was set for the recording of positive PLA signals (Bromfield et al., 2015b). Using this criterion, a total of 100 spermatozoa were assessed per sample (n = 5) and the percentage of PLA positive cells was recorded.
Statistics
Experiments reported in this study were repeated on at least five unique biological samples, with each sample representing semen collected from a single healthy normozoospermic donor. For the purpose of assessing acrosome reaction status and dynamin labelling profiles, ≥100 spermatozoa were counted in each sample and the corresponding percentage of acrosome reacted or positively labelled cells, respectively were determined dividing by the total number of cells counted. Graphical data are presented as the mean values ± SEM, which were calculated from the variance between samples. Statistical significance was determined by using an ANOVA.
Results
Detection of dynamin isoforms in human testis and mature spermatozoa
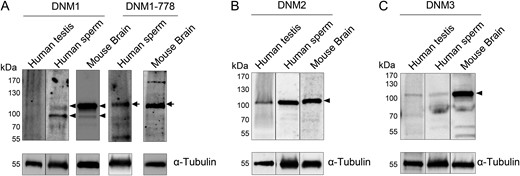
The presence of the three canonical dynamin isoforms (DNM1, DNM2 and DNM3) was assessed in lysates prepared from human testis and ejaculated spermatozoa. For this purpose, testis lysate was purchased from a commercial source (Santa Cruz Biotechnology) and Percoll fractionated spermatozoa were isolated from the semen of at least five donors of known fertility. In the case of DNM1, a predominant band of the anticipated size (~100 kDa) was detected in the mature sperm lysate (Fig. 1A). Notably however, an equivalent protein was not detected in human testis lysate (Fig. 1A). To discount the possibility of protein degradation within the testis lysate, these samples were further assessed by stripping of the membranes and re-probing with anti-α-tubulin antibody. This approach confirmed both the integrity of the testis protein sample and the equivalent protein loading achieved between testis and sperm lysates. To address the alternative possibility of relatively low DNM1 expression in human testes, the quantity of testis protein was increased three fold (i.e. 30 μg versus 10 μg for sperm lysate) but there was no associated increase in the detection of DNM1 (data not shown).
Identification of dynamin isoforms in human testis and sperm lysates. Human testis and sperm lysates were analysed for the presence of (A) dynamin 1 (DNM1), (B) dynamin 2 (DNM2) and (C) dynamin 3 (DNM3) using standard immunoblotting protocols. Mouse brain tissue homogenates were resolved alongside the human samples as a positive control for dynamin expression. After initial dynamin labelling, blots were stripped and re-probed with anti-α-tubulin antibody to confirm equivalent protein loading of each sample. (A) The detection of a doublet of ~100 kDa for DNM1 (arrowheads) prompted a further investigation of the potential for post-translational phosphorylation of this isoform. For this purpose, anti-phospho-DNM1-778 antibody was used to probe the same samples of mouse brain and human sperm lysates, revealing positive labelling of the higher molecular weight band (arrows). These experiments were repeated on spermatozoa obtained from five different healthy normozoospermic donors and representative immunoblots are shown. In contrast, testicular lysates were obtained from a commercial supplier and represent material prepared from a single healthy donor.
The detection of a doublet of around 100 kDa for DNM1 in sperm lysates (Fig. 1A, arrowheads) also prompted a further investigation of the potential for this band to represent a phosphorylated form of the protein. For this purpose, additional immunoblotting was conducted on the same homogenates of mouse brain (a tissue in which abundant phosphorylated DNM1 is present), and human spermatozoa using an antibody that detects phosphorylated DNM1 serine-778. Labelling with this antibody was restricted to the higher molecular weight band (Fig. 1A, arrows) potentially indicating that the basal level of DNM1 phosphorylation differs depending on the tissue from which the protein is isolated.
Notwithstanding some minor cross-reactivity, dominant protein bands corresponding to those of the predicted size for DNM2 and DNM3 (also ~100 kDa) were detected in both the human testis and sperm lysates (Figs 1B and 1C, arrowheads). In all cases, an additional positive control consisting of mouse brain lysate was subsequently probed for DNM1, DNM2 and DNM3, revealing the labelling of the same size band in this tissue (Fig. 1).
Immunolocalization of dynamin isoforms in human testis
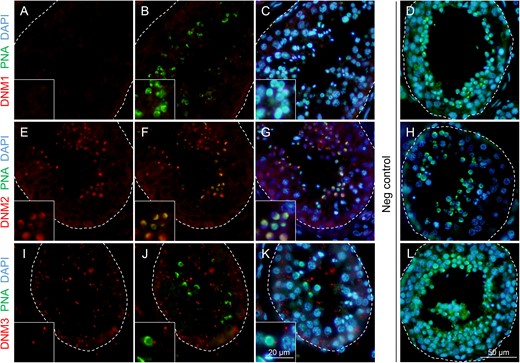
In view of the detection of dynamin expression in the human testis and/or sperm lysates, we next sought to investigate the specific localization of these enzymes within histological sections of human testes. Immunofluorescence was applied in this study in combination with a peanut agglutinin (PNA) counterstain of the developing sperm acrosome (Mortimer et al., 1987), and representative micrographs illustrating the expression patterns of dynamin are shown in Fig. 2. Consistent with the immunoblotting of testis lysate, immunofluorescence failed to reveal any significant DNM1 expression in human testis sections (Fig. 2A–C). The absence of DNM1 labelling persisted despite the application of a variety of antigen retrieval techniques in combination with trials to optimize incubation time and antibody concentration (data not shown). These data contrast those obtained for DNM2 immunolabelling, with distinct fluorescence foci being readily detected for DNM2 in the developing germ cell population of the human testes. While the quality of the testis sections precluded the precise determination of the temporal expression of DNM2, spatially, the protein was clearly co-localized with PNA in round and elongating spermatids (Fig. 2E–G). Such findings implicate DNM2 in acrosomal biogenesis during the later stages of the spermatogenic cycle. Additional DNM2 immunofluorescence was also detected, albeit considerably weaker, within the Sertoli cells of the seminiferous tubules. In the case of DNM3, a punctate pattern of localization was observed throughout the seminiferous tubules and, whilst this labelling did appear in the vicinity of the developing germ cells (Fig. 2I-K), we failed to detect any direct co-localization of DNM3 and PNA in germ cell populations. These later findings agree with those reported in the mouse testis (Reid et al., 2012), and suggest that DNM3, like that of DNM1, is unlikely to participate in the morphogenesis of the acrosomal vesicle in human spermatozoa. The specificity of all immunolabelling studies was confirmed through the inclusion of negative controls comprising secondary antibodies only (Fig. 2D, H, L) and anti-dynamin antibodies that had been pre-absorbed with excess immunizing peptide (where available, Supplementary Fig. S1A). As anticipated, none of these controls revealed any labelling of testis sections.
Immunofluorescence detection of dynamin isoforms within the human testis. Antibodies against (A, B and C) DNM1, (E, F and G) DNM2 and (I, J and K) DNM3 were used to determine the localization of these proteins (red) in human testis sections. These sections were subsequently counterstained with PNA (green) and DAPI to reveal the acrosome, and the cell nuclei (blue) of developing germ cells, respectively. For clarity, the structure of seminiferous tubules has been outlined. Among the three dynamin isoforms examined, only DNM2 was found to co-localize with the developing acrosomal vesicle. (D, H and L) The specificity of antibody labelling was confirmed through the inclusion of negative controls (Neg) in which antibody buffer was substituted for the primary antibody. These experiments were replicated on material from three human donors and representative immunofluorescence images are shown. Insets are of equivalent sections shown at higher magnification.
Immunolocalization of dynamin isoforms in human spermatozoa
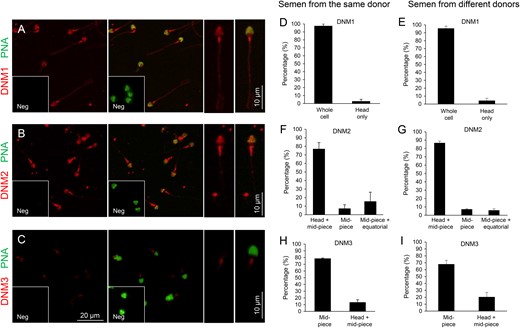
Immunofluorescent localization of dynamin was also performed on the isolated spermatozoa of normozoospermic donors (Fig. 3). In order to account for the possibility of either inter- and intra-donor variability in dynamin expression, these studies were conducted on spermatozoa obtained from at least five different donors, as well as on the spermatozoa purified from multiple ejaculates obtained from the same donor, respectively. Among the notable findings from this analysis was the detection of strong DNM1 labelling throughout the entire spermatozoon (Fig. 3A). Importantly, this pattern of localization was present in virtually all cells examined, irrespective of whether they were sourced from multiple ejaculates of the same, or different, donors (Fig. 3D and E). While such results clearly contrast those obtained in human testis sections (Fig. 2A–C), they nevertheless accord with the positive DNM1 protein band labelled in human sperm lysates (Fig. 1A). Since spermatozoa leaving the testis are incapable of either gene transcription or protein translation, such findings raise the prospect that DNM1 may be acquired by spermatozoa from the external environment in which they are bathed during their functional maturation in the epididymis.
Immunofluorescence detection of dynamin isoforms within human spermatozoa. Antibodies against (A) DNM1, (B) DNM2 and (C) DNM3 were used to determine the localization of these proteins (red), in mature human spermatozoa. The acrosomal domain of these cells was subsequently counterstained with PNA (green). Higher magnification images of the dominant labelling patterns for each antibody are depicted on the right of panels (A), (B) and (C), Neg, Negative control (secondary antibody only). (D–I) The distinct labelling patterns for each dynamin isoform were quantified, with 100 cells being examined per sample. These studies were replicated with spermatozoa isolated from three different ejaculates of the same donor (D, F and H) or single ejaculates from each of six different donors (E, G and I).
To begin to investigate this possibility, human epididymal sections were subjected to immunofluorescence labelling under identical conditions to those described for the testes. This analysis revealed positive DNM1 staining throughout the cytoplasm of the epithelial cells lining the epididymal duct (Supplementary Fig. S2A). Additional labelling was also detected in the lumen of the epididymis and, at least a portion of this, was found to co-localize with sperm heads (Supplementary Fig. S2A). Although we failed to source a reliable supply of human epididymal fluid, we were able to detect a positive signal for DNM1 by immunoblotting of whole seminal fluid (Supplementary Fig. S2B). A similar DNM1 band was also present in fractionated seminal fluid samples prepared using an OptiPrep density gradient protocol that has been optimized for the isolation of extracellular vesicles (Supplementary Fig. S2B). While further experimentation is required before definitive conclusions can be drawn, such data offer tentative support for the delivery of DNM1 to maturing human spermatozoa during their post-testicular maturation.
Similar to DNM1, DNM2 was also readily detected in human spermatozoa. In this case however, the most common pattern of labelling placed DNM2 in the sperm head and the mid-piece of their flagellum, with essentially no labelling being detected in the principal-piece of the flagellum (Fig. 3B, F and G). The labelling of DNM2 in the head was further verified by electron microscopy, which demonstrated positive immunogold labelling within the acrosomal domain (Supplementary Fig. S3). In contrast with DNM2, only very weak DNM3 staining was recorded in human spermatozoa. In the majority of cells, this labelling appeared restricted to the mid-piece of the flagellum, but in ~20% of the population it was accompanied by additional labelling within the sperm head (Fig. 3C, H and I). Such weak labelling of DNM3 raises the prospect that DNM1 and/or DNM2 fulfil the predominant functional role(s) of dynamin in mature spermatozoa. In all cases, dynamin labelling was highly reproducible and displayed minimal variation among the biological replicates examined. Similarly, the inclusion of negative controls in which spermatozoa were incubated with either secondary antibodies only (Fig. 3A–C, insets) or anti-dynamin antibodies that had been pre-absorbed with excess immunizing peptide (where available, Supplementary Fig. S1B) also supported the specificity of the immunofluorescent staining we documented across these samples.
Inhibition of dynamin 1 and 2 suppresses the induction of acrosomal exocytosis in vitro
The localization of DNM1 and DNM2 in the anterior region of the head of mature human spermatozoa ideally positions these two isoforms to participate in the regulation of acrosomal exocytosis, consistent with the putative function of the enzymes in mouse spermatozoa (Reid et al., 2012). We therefore investigated whether pharmacological inhibition of DNM1 and DNM2 could compromise the in vitro induction of acrosomal exocytosis in human spermatozoa. However, prior to implementing these experiments, we first sought to optimize an assay capable of eliciting an acrosome reaction independent of the use of a calcium ionophore. For this purpose, capacitated populations of human spermatozoa were primed with progesterone during incubation in capacitating BWW media formulated to encompass the pH range of 6.4–7.8. Live cells were subsequently assessed for their acrosomal status by labelling with FITC-conjugated PNA, with those cells displaying curled tails (induced by incubation in HOS medium) and green fluorescence over the complete peri-acrosomal domain being classified as acrosome intact (Cheng et al., 1996). In contrast, acrosome reacted spermatozoa were defined as those in which PNA was either absent or restricted to the equatorial domain (Cheng et al., 1996; Esteves et al., 1998). By imposing these criteria, we consistently achieved optimal acrosome reaction rates in BWW with a pH of 7.00–7.05 (Supplementary Fig. S4).
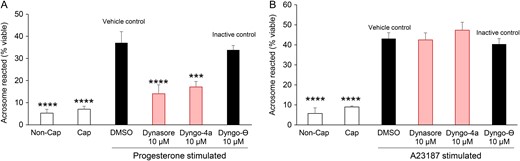
Using these optimized conditions, populations of human spermatozoa were found to be significantly inhibited in their capacity to undergo a progesterone-induced acrosome reaction if capacitation medium was supplemented with the dynamin inhibitors of Dynasore or Dyngo 4a (Fig. 4A, P < 0.05), compared to spermatozoa from the capacitated + vehicle (DMSO) control group. The inclusion of Dyngo-⊖, an inactive chemical analogue of Dynasore and Dyngo 4a, did lead to a modest suppression of acrosome reaction rates. Importantly however, this reduction did not prove to be significantly different from that of either of the positive control treatment groups (capacitated ± DMSO), but did differ significantly (P < 0.05) compared to both Dynasore and Dyngo 4a groups, thus precluding the possibility of non-specific pharmacological inhibition.
Examination of the effect of dynamin inhibition on the ability of human spermatozoa to engage in acrosomal exocytosis. PNA staining was applied to record the acrosomal status of viable spermatozoa from each of several treatment groups. Cells were induced to acrosome react via either a (A) progesterone (15 μM) or (B) calcium ionophore (A23187, 2.5 μM) stimulus. (A) A significant reduction in the number of acrosome reacted cells was observed in the presence of dynamin inhibitors (Dynasore or Dyngo, 10 μM) when compared to treatment with either the vehicle control (DMSO) or an inactive isoform control (Dyngo-⊖). (B) No such reduction was detected when acrosomal exocytosis was induced via A23187 challenge. Five biological replicates were conducted for each experiment and the results are presented as the means ± S.E.M. ***P < 0.001; ****P < 0.0001 compared to vehicle control (DMSO).
The selectivity of inhibition was reinforced by the demonstration that neither Dynasore nor Dyngo 4a could prevent the induction of acrosomal exocytosis by the calcium ionophore, A23187. Indeed, no significant differences were recorded in levels of A23187 induced acrosome reaction rates among the capacitated populations of spermatozoa treated with either Dynasore, Dyngo 4a, Dyngo-⊖ or DMSO (Fig. 4B). Further, neither of the dynamin inhibitors nor the inactive isoform control had a detrimental impact on sperm viability, which consistently remained above 75% in all treatment groups. In the case of Dyngo 4a, we did record a modest reduction in overall sperm motility (Supplementary Fig. S5). However, an equivalent reduction in sperm motility was not observed in the cells treated with Dynasore. Overall, these results are consistent with our previous reports of the response of mouse spermatozoa to dynamin inhibition (Reid et al., 2012), indicating a conserved relationship between the activity of this enzyme and the ability of spermatozoa to undergo a progesterone-induced acrosome reaction.
Comparison of dynamin expression in populations of good and poor quality human spermatozoa
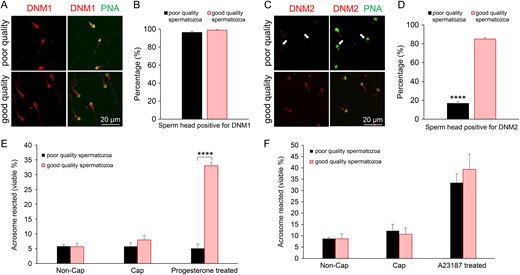
The ability to modulate acrosomal responsiveness in human spermatozoa through selective inhibition of dynamin prompted a more detailed evaluation of this enzyme in good and poor quality human sperm cells. To achieve this, semen samples from healthy normozoospermic individuals were fractionated via Percoll density gradient centrifugation into two discrete populations, referred to as a poor quality fraction (i.e. spermatozoa that partitioned at the interface of the 40–80% Percoll suspension) and a good quality fraction (i.e. high quality spermatozoa that pelleted at the base of 80% Percoll suspension). These two populations of human spermatozoa have been well characterized in previous reports and shown to possess significant differences in morphology (P < 0.03), motility and competence to fuse with the oolemma (P < 0.001) (Aitken et al., 1993). The presence of contaminating cellular debris, immature germ cells and other components within the poor quality fraction precluded the use of immunoblotting to accurately determine the relative amount of the dynamin isoforms in each subpopulation of spermatozoa. Thus, immunofluorescence was applied with a view to determining the percentage of spermatozoa harbouring DNM1 and DNM2 within the anterior head.
As illustrated in Fig 5A and B, a similar proportion of spermatozoa displayed DNM1 head labelling irrespective of whether they were recovered from the poor or good quality subpopulations. In marked contrast, a dramatic underrepresentation of DNM2 was recorded in spermatozoa that partitioned into the lower quality subpopulation. Notably, this highly significant reduction in DNM2 labelling was restricted to the sperm head, with the majority of these cells retaining DNM2 labelling in the mid-piece of their flagellum (Fig. 5C and D).
Comparison of dynamin expression in populations of good and poor quality human spermatozoa. Two subpopulations comprising good and poor quality spermatozoa were generated via Percoll density gradient centrifugation of semen from the same donor. (A and B) Immunolabelling of DNM1 revealed an equivalent staining pattern in both good and poor quality subpopulations of mature spermatozoa. (C and D) By contrast, immunolabelling of DNM2 demonstrated an apparent reduction in the amount of this protein in the head of poor quality spermatozoa (arrows) versus that of the good quality subpopulation of cells. Spermatozoa from each of the two subpopulations were induced to acrosome react with either (E) progesterone (15 μM) or (F) calcium ionophore (A23187, 2.5 μM) stimulus. (E and F) The poor quality spermatozoa were significantly compromised (P < 0.0001) in their capacity to complete a progesterone, but not an A23187, induced acrosome reaction compared to good quality spermatozoa. Non-Cap: the spermatozoa in these samples were not capacitated prior to the addition of progesterone or A23187. Cap: the spermatozoa in these samples were capacitated yet received no additional progesterone or A23187 stimulus. Five biological replicates were conducted with quantification of dynamin labelling and acrosomal status being performed across 100 cells per sample. Results are presented as the means ± S.E.M. ****P < 0.0001.
To begin to explore the functional consequences of reduced DNM2 expression in the head of poor quality spermatozoa, we compared the ability of our subpopulations to undergo an acrosome reaction in response to either a progesterone or calcium ionophore (A23187) stimulus. This analysis was again conducted using FITC-PNA labelling to assess acrosomal integrity and only live spermatozoa (possessing coiled tails as a result of incubation in HOS medium) were counted. Consistent with the reduction of DNM2 in the sperm head, those cells that were separated into the poor quality fraction proved entirely refractory to progesterone stimulus (Fig. 5E). Notably, the highly significant diminution in the response of these poor quality cells occurred despite the apparent retention of DNM1 in the sperm head (Fig. 5A and B). It also contrasted with the results obtained with the good quality subpopulation, over 35% of which were induced to acrosome react following progesterone challenge (Fig. 5E). Importantly, this lack of responsiveness was restricted to progesterone-induced acrosome reactions, such that no equivalent decrease was observed following exposure to calcium ionophore (A23187) (Fig. 5F).
CDK1 regulates the human sperm acrosome reaction through dynamin phosphorylation
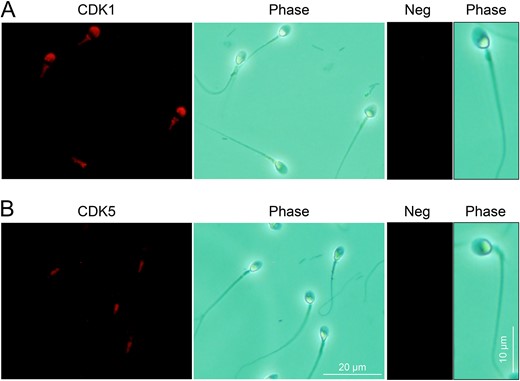
It is well established that dynamin function is regulated through alternating cycles of phosphorylation/de-phosphorylation. We therefore sought to investigate if DNM1 and DNM2 activity in human spermatozoa is also regulated through phosphorylation. Specifically, we focused on the characterization of CDKs (CDK1 and CDK5), serine/threonine kinases that reportedly target DNM2 Ser-764 and DNM1 Ser-778 for phosphorylation in somatic cells (Tan et al., 2003; Chircop et al., 2011). Through the use of immunofluorescence labelling, we found CDK1 was detected in the peri-acrosomal region of the human sperm head (Fig. 6A). In contrast, CDK5 displayed a restricted profile of localization within the mid-piece of the flagellum with no accompanying head labelling being detected irrespective of the functional status (non-capacitated, capacitated and progesterone treated) of the sperm samples analysed (Fig. 6B illustrates the representative labelling detected in non-capacitated spermatozoa). This raises the possibility of CDK1 targeting the substrates of DNM1 and/or DNM2 in the peri-acrosomal domain.
Immunofluorescence detection of cyclin dependent kinases (CDK1 and CDK5) in human spermatozoa. (A) Immunolabelling of human spermatozoa with anti-CDK1 antibodies revealed the strong labelling of the protein within the acrosomal and mid-piece domains. (B) Equivalent immunolabelling with anti-CDK5 antibodies demonstrated a restricted pattern of localization within the mid-piece of the flagellum and no accompanying head labelling. (A and B) The specificity of antibody labelling was confirmed through the inclusion of negative controls (Neg) in which antibody buffer was substituted for the primary antibody. Three biological replicates were conducted and representative images are shown.
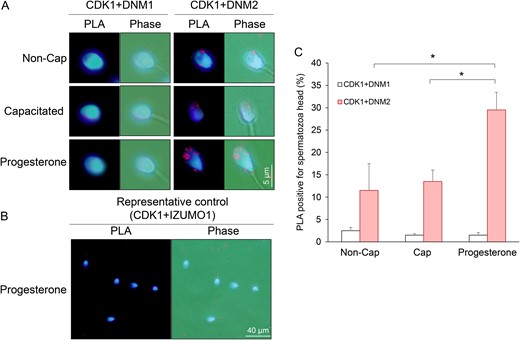
An in situ PLA was therefore employed to assess the co-localization of DNM1/DNM2 and CDK1 using the criterion of ≥3 punctate red fluorescent spots within the sperm head as a threshold for positive PLA labelling (Bromfield et al., 2015b) (Fig. 7). The combination of anti-CDK1 and anti-DNM2 antibodies, generated positive PLA signals that were primarily distributed over the anterior region of the sperm head. Although the percentage of CDK1/DNM2 PLA positive cells did not change following the induction of capacitation, it was significantly increased (from ~5 to ~30%, P < 0.01) upon the receipt of a progesterone stimulus. In contrast, minimal PLA labelling was detected for the combination of anti-CDK1 and anti-DNM1 antibodies irrespective of the functional status of the spermatozoa (Fig. 7A, C and Supplementary Fig. S6). Similarly, the irrelevant combination of anti-CDK1 and anti-IZUMO1 (Fig. 7B) or anti-DNM1 and anti-BAG6 antibodies (data not shown) also failed to generate positive PLA signals.
Analysis of CDK1/dynamin 1 and CDK1/dynamin 2 interaction in human spermatozoa. PLAs were applied to investigate the putative interaction between CDK1/DNM1 and CDK1/DNM2 in the anterior region of the human sperm head. This assay generates punctate red fluorescent signals when the targeted pair of proteins reside within a maximum of 40 nm from each other. A threshold of ≥3 red fluorescent spots within the sperm head was set for recording of positive PLA labelling. For clarity, cells were counterstained with the nuclear stain, DAPI. (A) Representative PLA images demonstrating that CDK1 resides in close proximity to DNM2, but not DNM1, in the anterior region of the sperm head. (B) The specificity of this interaction was confirmed through the use of antibodies targeting IZUMO1, a protein that is not expected to be a substrate of CDK1. (C) Data from this assay were quantified by recording the percentage of PLA positive spermatozoa, with 100 cells being examined per sample. Experiments were conducted on five biological replicates and the results are presented as the means ± S.E.M. *P < 0.05 compared to progesterone treatment (CDK1/DNM2).
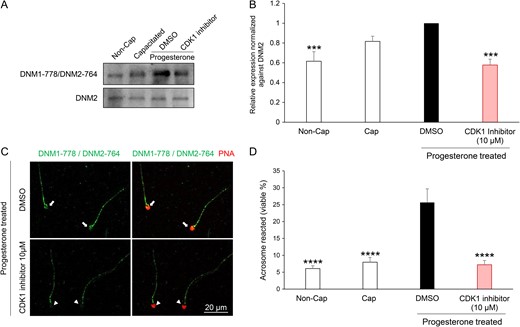
Having secured evidence for a putative interaction between CDK1 and DNM2, we next sought to investigate whether pharmacological inhibition of CDK1 could compromise phosphorylation of DNM2 and consequently result in reduced rates of acrosomal exocytosis following progesterone stimulation. Previous reports indicate that the dominant site for DNM2 phosphorylation site is the residue Ser-764, which is homologous to the phosphorylation site of Ser-778 in DNM1 (Chircop et al., 2011). Thus, we treated human spermatozoa with 10 μM CDK1 inhibitor (IC50 = 5.8 μM; Andreani et al., (2000)) prior to assessing DNM1 Ser-778/DNM2 Ser-764 phosphorylation status with an antibody (anti-DNM1 Ser-778) that also recognizes the phosphorylated form of DNM2 Ser-764 residue (Supplementary Fig. S7). As shown in Fig 8A and B, spermatozoa treated with the CDK1 inhibitor experienced a significant reduction in the levels of phosphorylated DNM1 Ser-778/DNM2 Ser-764. Importantly, this reduction in phospho-labelling was restricted to the sperm head, coinciding with the location of CDK1 (Supplementary Fig. S8), and was not attributed to the loss of acrosomal contents, which were readily labelled with PNA in these spermatozoa (Fig. 8C). (Loss of acrosomal contents has also been associated with loss of DNM1 Ser-778/DNM2 Ser-764 in the acrosomal region; Supplementary Fig. S9). The impact of CDK1 inhibition also extended to a significant reduction in the rates of acrosomal exocytosis elicited in response to progesterone challenge (Fig. 8D) compared to that of the vehicle control (DMSO) group. Taken together, these data implicate the necessity for dynamin phosphorylation in the regulatory control of human sperm acrosomal exocytosis.
Pharmacological inhibition of CDK1 reduces dynamin phosphorylation and the ability of human spermatozoa to acrosome react. (A) Immunoblotting of lysates prepared from human spermatozoa incubated with CDK1 inhibitor (10 μM) revealed a reduction in the progesterone stimulated phosphorylation of DNM1-778/DNM2-764 residues compared to that cells treated with the vehicle alone (DMSO). Blots were stripped and re-probed with anti-DNM2 antibody to confirm equivalent levels of DNM2 in each sample. (B) The intensity of DNM1-778/DNM2-764 labelling was quantified by Image J and normalized to DNM2 levels. (C) Immunofluorescent labelling of spermatozoa confirmed that the reduction in DNM1-778/DNM2-764 phosphorylation was restricted to the anterior (acrosomal) region of the sperm head (coinciding with the location of CDK1). (D) CDK1 inhibition also led to a significant reduction (P < 0.01) in the number of acrosome reacted cells following treatment with progesterone when compared to the vehicle only control (DMSO). All experiments were conducted on five biological replicates and the results are presented as the means ± S.E.M. ***P < 0.001; ****P < 0.0001 compared to DMSO vehicle control.
Discussion
The completion of an acrosome reaction is a prerequisite for successful fertilization and accordingly, failure to complete this unique exocytotic event represents a common aetiology underpinning the defective sperm function of infertile males (Muller, 2000; Liu and Baker, 2003). In support of recent studies implicating the dynamin family of mechanoenzymes as important regulators of the acrosome reaction in murine spermatozoa (Reid et al., 2012), we have provided evidence that this function may also be conserved in the spermatozoa of our own species. Specifically, our study has revealed that the canonical dynamin isoforms of DNM1, DNM2 and DNM3 are each represented among the proteome of ejaculated human spermatozoa. Further, DNM1 and DNM2 are ideally positioned within the anterior portion of the sperm head to enable them to exert an important regulatory influence over the acrosomal status of human spermatozoa. Accordingly, we report that the loss, or pharmacological inhibition of this enzyme, is associated with a concomitant reduction in the ability of human spermatozoa to complete a progesterone-induced acrosome reaction. The selectivity of such inhibition was supported by absence of an equivalent impact on acrosomal exocytosis induced by the calcium ionophore, A23187, which has the ability to bypass key physiological mechanisms required for acrosomal exocytosis (Buffone et al., 2014).
The presence of dynamin isoforms within human spermatozoa has previously been recorded among the inventories arising from large scale proteomic profiling studies of fertile donors (Wang et al., 2013; Amaral et al., 2014). Indeed, these studies have revealed that human sperm harbour dynamin 2 and 3, in addition to the dynamin-like isoforms such as dynamin 1 like protein, dynamin-like 120 kDa protein S1. However, to the best of our knowledge, our study represents the first to confirm the presence of the three canonical dynamin isoforms and report their spatial expression profiles in the human testis, epididymis and ejaculated spermatozoa. Our findings raise the prospect that this family of mechanoenzymes may fulfil diverse, yet evolutionary conserved roles in the support of male germ cell development and function. Notwithstanding this possibility, we also observed some variation in the expression patterns of dynamin isoforms anticipated on the basis of previous observations in rodent models.
One particular curiosity was our inability to detect appreciable levels of dynamin 1 in human testis sections despite the relatively strong labelling of the protein in ejaculated spermatozoa (confirmed by both immunofluorescence and immunoblotting). Among the possible explanations for this observation is that DNM1 is either unmasked, or alternatively, uniquely acquired during the post-testicular maturation of spermatozoa. Arguing against the former explanation is the fact that we were unable to detect DNM1 in testis sections despite the application of a variety of antigen retrieval steps that have previously been successfully implemented in the mouse (Zhou et al., 2017). Further, transcript and protein expression data sourced from public databases (Gene Expression Omnibus profile and the European Bioinformatics Institute) also indicate that DNM1 is not expressed in the human testes. On the other hand, the tenet that DNM1 may be transferred to maturing spermatozoa draws on a wealth of evidence that the sperm proteome is substantially modified during their transit of the epididymis (Dacheux et al., 2006, 2016; Sullivan and Mieusset, 2016). One of the principle mechanisms implicated in this form of intercellular communication is the interaction formed between spermatozoa and a population of extracellular vesicles, known as epididymosomes, which are secreted into the epididymal lumen (Sullivan, 2015). In support of this mechanism, we detected DNM1 in whole seminal fluid as well as an extracellular vesicle fraction isolated from this material. While the validity of this model of DNM1 acquisition awaits further investigation, the labelling of the protein in the peri-acrosomal domain of ejaculated spermatozoa suggests that it may work in tandem with DNM2, which occupies a similar location, to regulate the release of the acrosomal contents during a progesterone-induced acrosome reaction. Such functional redundancy is a relatively common feature of dynamin expression in somatic cells (Altschuler et al., 1998), and is also commensurate with the overall importance of the acrosome reaction in terms of securing successful fertilization.
Notwithstanding the potential for overlapping function between DNM1 and DNM2, our collective data lead us to conclude that DNM2 is likely to fulfil the dominant role in terms of both male germ cell development and function. Indeed, the conditional ablation of this ancestral form of the enzyme, which is most closely related to the dynamin-like proteins detected in lower organisms (Chircop et al., 2011), leads to a complete arrest of early germ cell development (Redgrove et al., 2016). In contrast, the loss of DNM1 at a similar stage of germ cell development had no discernible effect on either germ cell development or the fertility of the null males (Redgrove et al., 2016). It is also noted that during latter phases of germ cell development, the DNM2 protein co-localizes with PNA at a time when Golgi-derived vesicles begin to fuse to form a single large secretory vesicle that eventually gives rise to the acrosome (Kierszenbaum et al., 2003). This agrees with data implicating DNM2 as a key regulator of newly formed vesicles and post-Golgi vesicle exocytosis in various somatic cell types (Jones et al., 1998; Kreitzer et al., 2000; Grimmer et al., 2005; Kessels et al., 2006; Jaiswal et al., 2009). On the basis of similar patterns of localization in human testis, it is tempting to suggest that DNM2 may fulfil an equivalent role in our species. In a similar vein, the expression of DNM2 detected within the epididymal epithelium suggests that the protein may also exert an influence over the secretion of proteins into the epididymal lumen and, in so doing, influence the maturation of spermatozoa (Zhou et al., 2017).
The possibility that such functional conservation extends to the control of mature human spermatozoa is supported by selective pharmacological inhibition, which led to a significant reduction in the ability of these cells to complete an acrosome reaction. However, in seeking to discern the relative importance of DNM1 and DNM2 in this response, it is notable that the dynamin inhibitors employed in this study target both isoforms with equivalent efficacy owing to their selective inhibition of the GTPase activity of these enzymes (Macia et al., 2006; McCluskey et al., 2013). Despite this, the importance of DNM2 is underscored by the dramatic underrepresentation of this isoform, but not DNM1, in the subpopulation of poor quality spermatozoa recovered from healthy donors following density gradient centrifugation. Importantly, the apparent loss of DNM2 was correlated with reduced ability of these cells to complete a progesterone-induced acrosome reaction. The fact that these cells retained the competence to complete a calcium ionophore induced acrosome reaction, which is relatively forgiving of the functional status of the cell, indicates that this was not associated with compromised structural integrity of the acrosomal vesicle. While such findings would clearly benefit from further validation across an expanded cohort of patients with zona pellucida penetration defects, they nevertheless support the importance of the DNM2 in governing the acrosomal responsiveness of human spermatozoa.
An important caveat to this interpretation is that the putative importance of progesterone as a physiologically relevant stimulus to prime human spermatozoa for induction of acrosomal exocytosis remains the subject of active debate (Buffone et al., 2014). Recent evidence mounts a compelling case in favour of this hypothesis by revealing that progesterone can interact either directly (Lishko et al., 2011; Strünker et al., 2011), or indirectly with CATSPER channels in human spermatozoa (Miller et al., 2016). In the latter model, it is suggested that progesterone binds to abhydrolase domain containing 2 (ABHD2). This lipid hydrolase is highly expressed in human spermatozoa where it is responsible for the degradation of endocannabinoid 2-arachidonoylglycerol (2AG), and hence its depletion from the plasma membrane. Since 2AG inhibits CATSPER, its progesterone-dependent removal (Miller et al., 2016) serves to modulate intracellular calcium levels as necessitated for the induction of acrosomal exocytosis (Beltrán et al., 2016). However, the fact that both CATSPER channels and ABHD2 are restricted to the flagellum of human spermatozoa (Lishko et al., 2011; Strünker et al., 2011; Miller et al., 2016) raises the question of whether the CATSPER initiated calcium fluxes could be propagated to the anterior region of the sperm head. Tentative support for this model rests with the demonstration that mouse spermatozoa challenged with solubilised ZP proteins responded via the propagation of a CATSPER-dependent calcium wave that progressed from the flagellum toward the head (Ren and Xia, 2010). An alternative possibility is that progesterone could induce acrosomal exocytosis via interaction with progesterone receptors (Aquila and De Amicis, 2014) that are known to be located in the mid-piece/neck region of human spermatozoa (Thomas et al., 2009). A further consideration is the prospect that the spermatozoa of different species may respond differently to progesterone stimulus. For instance, unlike their human counterparts, the CATPSER harbored by mouse spermatozoa is reportedly refractory to progesterone stimulus. Similarly, 2AG is removed from the plasma membrane of mouse spermatozoa prior to their completion of epididymal transit (Miller et al., 2016). Despite this, mouse spermatozoa do retain the capacity to respond to progesterone through induction of an acrosome reaction (Osman et al., 1989).
Irrespective of the pathways employed, our data emphasize the importance of downstream events tied to progesterone stimulus via the demonstration that dynamin phosphorylation can promote the induction of acrosomal exocytosis in the spermatozoa both humans and mice. Indeed, in our hands progesterone challenge led to an increase in phosphorylation of DNM1 Ser-778/DNM2 Ser-764 in human spermatozoa. The consequences of the phosphorylation of these dynamin residues has been studied extensively in the context of membrane remodelling and vesicle scission in somatic cells. In neural tissue, replacement of DNM1 and DNM3 with DNM1 dephospho- or phospho-mimetic mutations can either accelerate or decelerate the endocytotic process (Armbruster et al., 2013). Similarly, DNM2 phosphorylation in endothelial cells has proven to be essential for scission of caveolae (Shajahan et al., 2004). In our own studies, we have previously reported a substantive increase in dynamin phosphorylation in the peri-acrosomal region of mouse spermatozoa following progesterone challenge (Reid et al., 2012). On the basis of these data we hypothesized that dynamin phosphorylation may slow the rate of expansion and/or stabilize the formation of fusion pores between the outer acrosomal membrane and plasma membrane thereby prolonging the exocytosis process consistent with the protracted kinetics of this unique secretory process. Our data from this study suggest that dynamin phosphorylation may also play a pivotal role in the modulation of its function in human spermatozoa. Further work is now needed to reconcile the differences in progesterone action in the spermatozoa of these two species.
In order to initiate these studies, we sought to identify the kinase responsible for dynamin phosphorylation, with our data revealing that CDK1 is a likely candidate in the phosphorylation of DNM2, but not DNM1, within the acrosomal region of progesterone stimulated spermatozoa. This result is consistent with independent studies identifying CDK1 as the principle kinase responsible for DNM2 phosphorylation in somatic cells and in mouse germ cells (Chircop et al., 2011; Ferguson and De Camilli, 2012; Redgrove et al., 2016). While we sought to confirm the CDK1/DNM2 interaction using reciprocal co-immunoprecipitation, regrettably this interaction proved recalcitrant to this strategy irrespective of whether CDK1 or DNM2 were used as the bait. Among the possible explanations, we consider that the transient nature of the kinase/substrate interaction, which is typically measured in milliseconds, precluded the capture of this event. Nevertheless, we did confirm that CDK1 co-localizes with DNM2 in the anterior region of the sperm head and that the selective inhibition of this kinase suppressed the ability of these cells to respond to progesterone challenge in terms of both DNM2 phosphorylation (at Ser-764) and acrosomal exocytosis. In accounting for these findings, it is noteworthy that dynamin phosphorylation is known to regulate the enzymes GTPase activity and hence the scission of vesicles in neuronal tissues (Robinson et al., 1993). These findings encourage speculation of a causative link between the suppression of DNM2 Ser-764 phosphorylation and the GTPase-dependent conformational change in the protein that putatively regulates acrosomal exocytosis. However, we are cognizant that CDK1 has the potential to phosphorylate multiple substrates (Ubersax et al., 2003) and we remain uncertain what, if any, role(s) these additional targets have in the regulation of sperm function; thus, these data must be interpreted with caution.
In summary, the collective findings described in this, and our preceding studies of dynamin function, lead us to propose that this family of mechanoenzymes may hold conserved roles in the regulation of several aspects of male fertility. Chief among these are the support of acrosome formation during germ cell development and the regulated exocytotic release of the acrosomal contents that precedes fertilization. Such activity appears to be intimately tied to the phosphorylation status of the protein, with our current study identifying CDK1 as a key regulatory kinase in the phosphorylation of DNM2. Our study has also provided evidence that the underrepresentation of DNM2 is associated with poor quality spermatozoa that fail to complete acrosomal exocytosis. Although the causative nature of these phenomenon awaits further investigation, this study stimulates further research into the potential of dynamin as a novel molecular target to assist with diagnosis of male infertility and the establishment of discrete criteria for the stratification of infertility patients into appropriate therapeutic options.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Acknowledgements
The authors are grateful to Jodie Powell for orchestrating the panel of healthy donors used in this research and Dr Zhuo Yu of Shanghai Jiaotong University for the supply of human testis and epididymis sections.
Authors’ roles
W.Z. conducted the experiments and generated the manuscript. A.L.A. provided technical assistance. A.P.T conducted the electron microscopy experiment. G.N.D.I. and E.A.M. contributed to study conception and design, data interpretation and manuscript editing. A.M. provided Dyngo-⊖, the instruction of the usage of dynamin inhibitors. B.N. conceived this study and contributed to study design, data interpretation, and manuscript preparation. All authors approved the final version and submission of this article.
Funding
National Health and Medical Research Council of Australia Project Grant (APP1103176 B.N. and E.A.M).
Conflict of interest
The authors declare that they have no conflicts of interest.