-
PDF
- Split View
-
Views
-
Cite
Cite
Ferran Bossacoma Busquets, Antoni Noguera-Julian, Emilia Sanchez, Claudia Fortuny, Dolutegravir plus abacavir/lamivudine works in adolescents, but size matters, Journal of Antimicrobial Chemotherapy, Volume 72, Issue 10, October 2017, Pages 2958–2960, https://doi.org/10.1093/jac/dkx235
Close - Share Icon Share
Sir,
We read with great interest the article by Briand et al.1 about the efficacy and safety of dolutegravir-based combined ART (cART) in 50 perinatally HIV-infected adolescents in France. We would like to share our experience with a once-daily fixed-dose combination (FDC) comprising dolutegravir (50 mg), abacavir (600 mg) and lamivudine (300 mg) (ViiV Healthcare, UK), in a series of HIV-infected adolescents in a referral paediatric hospital in Barcelona, Spain.
Between September 2015 and January 2017, dolutegravir FDC treatment was initiated in 12 HIV-infected adolescents [8 females; median (IQR) age = 16.4 (14.5–16.7) years], as a simplification strategy (n = 6), because of virological failure due to poor adherence (below 95%; n = 5) or as first cART (n = 1). The subjects had mainly acquired HIV infection perinatally (n = 10), half were of Caucasian origin, three had AIDS and all had previously tested negative for HLA-B*5701. Previous cART consisted of a PI-based, a raltegravir-based and a non-nucleoside-based regimen in seven patients, three patients and one patient, respectively; the median (IQR) number of daily tablets was 3 (3–5).
All patients and parents were satisfied with the treatment switch to a single daily tablet, yet they complained about the tablet size; six of them reported having to crush or split the tablet in order to swallow it. Regarding tolerance, two girls (aged 14.5 and 16.7 years) developed mild self-limited mood swings (oppositional and defiant disorders, grade 2/moderate psychiatric adverse events2) during the first weeks of treatment that did not lead to cART interruption; further, the treatment was well tolerated. No other clinical adverse events, new AIDS-defining events or deaths occurred during follow-up.
Median (IQR) follow-up on dolutegravir FDC treatment was 15.2 (14.4–15.9) months. No significant changes in median values of CD4 cell counts or metabolic parameters2 were observed over time. At baseline, only one 16.5 year old boy had a CD4 cell count <200/mm3; he properly adhered to cART and showed suppressed viral replication. His CD4 cell count had increased to 220 cells/mm3 after 16 months of treatment.
Viral suppression was observed in all patients who underwent cART simplification, in two switched due to virological failure and in the naive patient. Adherence to cART remained sub-optimal in three adolescents who were not able to control viral replication (7700, 3510 and 384 RNA-HIV copies/mL after 20, 19 and 13 months of treatment, respectively). In the latter cases, no integrase inhibitor resistance-associated mutations were identified in WGS analysis (GS Junior 454 Sequencing System, Roche, Spain).
Integrase inhibitor-based cART treatments are currently considered among the preferred regimens for the initial treatment of HIV infection in adults.3,4 Dolutegravir-, raltegravir- and elvitegravir-based regimens are also first-line drugs in adolescents in the USA,3 but they remain alternative options in Europe.5 All recommendations agree that once-daily cART regimens and those with low pill burden should be prescribed when feasible to maximize adherence.3–5 Perinatally infected adolescents are known to have poorer adherence to cART when compared with younger children.6 In this context, the availability of one-pill, once-daily FDCs of integrase inhibitor-based cART is very welcome. The use of dolutegravir plus abacavir/lamivudine (ViiV Healthcare, UK), and elvitegravir plus cobicistat, emtricitabine and tenofovir alafenamide (Gilead Sciences, UK) has been approved for adolescents aged over 12 years, while elvitegravir plus cobicistat, emtricitabine and tenofovir (Gilead Sciences, UK) is currently licensed only for adults in Europe.
As compared with the study of Briand et al.,1 in which the patients were older, in our series the main indication for switching to dolutegravir FDC was treatment simplification, and median duration of follow-up was longer. Dolutegravir-based cART regimens achieved good efficacy results in both cases: 78% in the series of Briand et al.1 and 75% in our series. It is notable that salvage therapy including dolutegravir attained viral suppression in 19/33,1 and 2/5 adolescents switched because of viral failure in our unit, similar to the previous results of the IMPAACT P1093 study in 23 adolescents.7 When HIV viraemia remained detectable, no new selection of resistance-associated mutations was observed in any of the patients in both series. This result is attributable to the high genetic barrier to resistance of dolutegravir.3
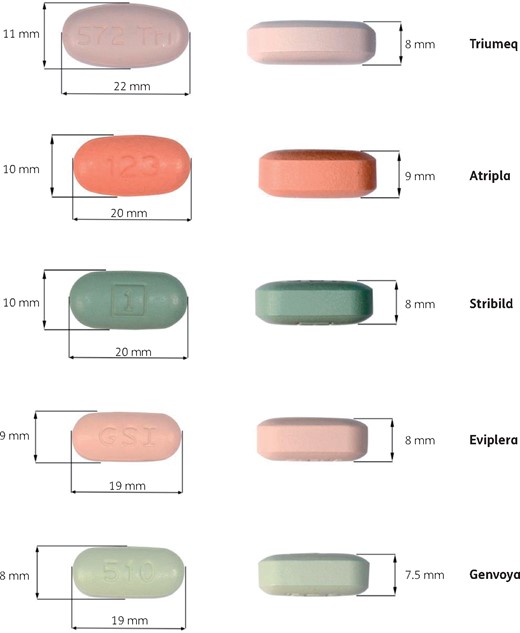
Pill-swallowing difficulties and the need for training are not uncommon in HIV-infected children and adolescents.8 This was not reported in the French study,1 where only 9/50 patients received dolutegravir FDC, but it affected all the patients in our series. Dolutegravir FDC is the largest of the currently available one-pill, once-daily FDCs for the treatment of HIV infection (Figure 1). This has not been previously reported, but may represent a barrier to adherence in the youngest adolescents and in adults presenting with comorbidities that affect swallowing function.9
One-pill, once-daily FDCs for the treatment of HIV infection: Triumeq (dolutegravir plus abacavir and lamivudine), Atripla (efavirenz plus emtricitabine and tenofovir), Stribild (elvitegravir plus cobicistat, emtricitabine and tenofovir), Eviplera (rilpivirine plus emtricitabine and tenofovir) and Genvoya (elvitegravir plus cobicistat, emtricitabine and tenofovir alafenamide). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
A recent study including three data sources in adults reported psychiatric symptoms (mainly insomnia, anxiety and depression) to be as common with dolutegravir-based regimens as with other cART combinations,10 mild to moderate in intensity and rarely leading to treatment discontinuation. Only suicidality is identified as a serious event. We clearly observed moderate and self-limited mood changes upon dolutegravir FDC treatment implementation in two young girls in our series, similar to what was reported in one French patient.1 In spite of the anecdotal evidence, dolutegravir-associated psychiatric toxicity might be related to younger age or higher dosage per kg of bodyweight. Considering the high rate of psychiatric disorders among perinatally HIV-infected adolescents,11 close psychological follow-up of children and adolescents receiving dolutegravir seems advisable.
Funding
This study was conducted as part of our routine work.
Transparency declarations
None to declare.
References
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.