-
PDF
- Split View
-
Views
-
Cite
Cite
Bart J. Rijnders, Eric Van Wijngaerden, Stefaan J. Vandecasteele, Marguerite Stas, Willy E. Peetermans, Treatment of long-term intravascular catheter-related bacteraemia with antibiotic lock: randomized, placebo-controlled trial, Journal of Antimicrobial Chemotherapy, Volume 55, Issue 1, January 2005, Pages 90–94, https://doi.org/10.1093/jac/dkh488
Close - Share Icon Share
Abstract
Objectives: The use of an antibiotic lock (AB-lock) for the treatment of catheter-related bloodstream infection (CRBSI) has been suggested, but randomized trials have never been performed.
Methods: A randomized, blinded, multicentre trial was set up to compare an AB-lock—containing vancomycin for Gram-positive or ceftazidime for Gram-negative bacteria—with placebo, in addition to parenteral AB therapy. We included only CRBSI from a long-term intravascular device (LTID) whether tunnelled or totally implanted.
Results: During 30 months, 174 patients with an LTID and bacteraemia were evaluated, of whom 85 had a CRBSI. Forty-six patients were included. Frequent reasons for exclusion were: catheter not vacant for >8–12 h/day for the AB-lock (n =10); yeast infection or mixed Gram-positive/negative infections (n =13); catheter removal preferred by the treating physician (n =7); and CRBSI <14 days after insertion or pocket/tunnel infection (n =10). Forty-four patients met the criteria for modified intention-to-treat analysis. The primary endpoint was failure to cure the CRBSI or relapse with the same strain. On study day 180 by Kaplan–Meier analysis, this occurred in 33% (seven of 21) in the AB-lock arm and in 57% (13 of 23) in the placebo arm (hazard ratio 0.55, P =0.10). A relapse with the same strain occurred in 9/23 with the placebo and 3/21 with the AB-lock (P =0.06).
Conclusion: Future studies should take into account the barriers to the use of AB-lock observed in this study. Most importantly, shorter lock dwell times and broader spectrum locks (e.g. antiseptic) should be investigated to target a larger patient population.
Introduction
Long-term intravascular devices (LTID) are routinely used in the management of oncology, haematology, haemodialysis, short-bowel and many other patients. One of the most frequently encountered complications is catheter-related bloodstream infection (CRBSI) and it has been estimated that about one-third of these infected catheters have to be removed even when an adequate parenteral antibiotic treatment is given.1 Some physicians use antibiotic-lock therapy (AB-lock) in addition to the parenteral administration of antibiotics. This mostly consists of a mixture of an antibiotic and heparin, with which the catheter is filled (‘locked’) when it is not in use. In this way, the concentration of the antibiotic inside the catheter can be orders of magnitude higher than the concentration reached during conventional treatment. Furthermore, these concentrations can remain in place for up to 24 h (when the catheter is not in use). Good evidence is available to support the AB-lock in the prevention of CRBSI in neutropenic patients, with several randomized, controlled trials proving its effectiveness in this setting.2–4 Since the first description of the AB-lock for the treatment of CRBSI, the technique has been greeted with great enthusiasm.5 Recently published guidelines on the management of catheter-related infections are in favour of the use of an AB-lock for the treatment of CRBSI from an LTID.1 The evidence supporting the use of an AB-lock for the treatment of CRBSI is, however, still fragmentary as only non-randomized observational studies with historical controls have been described. Success rates vary considerably. We performed a randomized, double-blind, placebo-controlled study of the AB-lock in the treatment of CRBSI in patients with an LTID.
Methods
The study was a randomized, multicentre, double-blind, placebo-controlled study. All patients hospitalized (adults as well as children) with an LTID in place and with a suspected catheter-related bacteraemia were eligible for inclusion. The patients were recruited from three hospitals in Belgium (one tertiary care hospital and two secondary care hospitals) where an estimated 1500 LTID (1000 totally implanted ports and 500 tunnelled catheters) are inserted per year. Participating units were three haematology units (one paediatric), two oncology units, one gastroenterology unit and three haemodialysis units. The institutional review board at each hospital approved the protocol, and all participants (or parents/guardian when appropriate) provided written informed consent.
To be eligible for the study, the following inclusion criteria had to be fulfilled. (1) Differential time to positivity (DTTP) of >2 h of blood cultures taken through a peripheral vein puncture and through the catheter.6 (2) When no peripheral blood cultures were available, patients could only be included when signs of sepsis were clearly associated in time with flushing of the catheter in patients in whom the catheter was only used on an intermittent basis (e.g. chills and fever during the time of, or immediately after, intermittent haemodialysis or flushing of the catheter). For CRBSI with coagulase-negative staphylococci, corynebacteria and propionibacteria (bacteria typically present in skin flora) at least two blood cultures taken at different times had to be positive. (3) As peripheral blood cultures are rarely available in paediatric oncology patients, inclusion of these patients was also allowed when they had fever >37.9°C, the chest X-ray and urine analysis was unrevealing, no possible clinical focus other than the catheter was apparent and the patient was not neutropenic. In these patients, at least two blood cultures—taken through the catheter at different time points and within 72 h—had to be positive. Bacteria were identified with standard microbiological methods, and using the VITEK system (bioMérieux) for Gram-negative bacteria and coagulase-negative staphylococci. For streptococci, the API Strep (bioMérieux) was used and for non-albicans Candida the ID32C (bioMérieux) system.
Patients with one of the following exclusion criteria were not allowed to participate. (1) Documented or suspected IgE-mediated allergy to vancomycin or β-lactam antibiotics, unless the patient was already receiving this antibiotic for systemic therapy. (2) Catheter removal planned within the following 2 weeks. (3) The antibiotic treatment was not to be given through the catheter. This latter exclusion criterion was needed because no active control was used except for a placebo. Therefore, in the placebo group, at no time would an antibiotic be present in the catheter of included patients when they were not receiving systemic antibiotic treatment through the catheter. (4) The catheter was in place for <2 weeks or (5) pocket, tunnel or exit-site infection, because in all these instances an extraluminal infection was more likely. (6) Complicated CRBI (endocarditis, osteomyelitis, septic thrombophlebitis). (7) Candidaemia. (8) Estimated patient survival <4 weeks. (9) Systemic antibiotic treatment active against the cultured bacteria had been started >72 h earlier. (10) The patient will not be an inpatient or attend the hospital daily for the 7 days after inclusion. (11) Previous inclusion into the study. (12) Administration of the AB-lock for a minimum of 8–12 h/day is not possible for at least the first 7 days after inclusion. (13) Triple lumen catheters. (14) CRBSI with a microorganism not susceptible to vancomycin or ceftazidime.
Preparation and use of AB-locks
All patients received systemic antimicrobial therapy through the catheter according to the Infectious Diseases Society of America (IDSA) guidelines.1
In addition, all patients received an AB-lock or placebo-lock. The hospital pharmacist of the university hospital of Leuven made 25 5 mL syringes with antibiotic- or placebo-lock at day 0 and again at day 7 because full antibiotic activity of the AB-lock is retained for only 7 days. The syringes were filled with a heparin solution (100 IU/mL) as placebo, or with a solution of heparin (100 IU/mL) with ceftazidime (for Gram-negative bacteria) or vancomycin (for Gram-positive bacteria) both at a concentration of 500 mg/L. Preparation of AB-locks and placebo was carried out under laminar airflow. The concentrations of the antibiotics and heparin mentioned above were chosen because data on stability and duration of antibacterial efficacy are available at these concentrations for different temperatures (room and body temperature) and up to 10 days.7 The AB-locks were used daily for a minimum of 7 days and a (preferred) maximum of 14 days (this, in general, is when the parenteral systemic antibiotic treatment is stopped). The catheter was filled with the AB-lock or placebo whenever not in use.
Endpoint
The primary endpoint used in this study was failure to cure the CRBSI. Failure to cure (=treatment failure) was defined as any of the following: (1) Catheter removal for whatever reason, except for removal because the catheter is no longer needed. Examples are: removal for persistent infection, or relapse of infection during or after treatment, but also removal as a consequence of occlusion of the catheter. (2) Relapse of bacteraemia with the same phenotypic strain (same species and antibiogram) with the catheter as the only focus of infection and for which antibiotic treatment was administered. (3) Death during the initial AB-lock treatment phase, whatever the cause. No distinction between causes of death (CRBSI or other) were made and so all deaths during the treatment phase were counted as failures because it would be impossible to ascertain, with certainty, whether CRBSI had or had not contributed to the death of the patient. (4) Death due to catheter infection at any time during the 6 month follow-up. To comply with this endpoint, the treating physician was asked if the CRBSI was the probable cause of death for every patient who died during the follow-up.
If, at the time of the death of a patient or at the time of removal of the catheter (because it was no longer needed) no relapse of bacteraemia had been observed, the primary endpoint was not reached. Prevention of the primary endpoint until 24 weeks after inclusion, death of the patient due to underlying disease or removal of the catheter (because no longer needed) was, in our opinion, the most clinically relevant outcome measure.
Patient follow-up was up to 24 weeks, catheter-removal (because no longer needed) or death of the patient (whichever came first). The following baseline data were collected: age, underlying disease, maximum body temperature during the previous 48 h, date of catheter insertion, type of catheter, neutrophil count, catheter used for parenteral nutrition or not and DTTP. During the days that the patient was receiving the study treatment, the time the catheter was locked with the study solution was recorded.
Randomization, allocation concealment and blinding
The allocation sequence was located at the clinical pharmacy of the university hospital during the time of the trial and only the research pharmacists had access to it. Pharmacists, of whom none was a co-investigator, performed patient randomization and preparation of syringes with placebo or AB-locks. Computer-generated random number tables were used. Randomization was conducted in blocks of four and stratified according to bacteria cultured (Gram-positive or Gram-negative). As vancomycin/heparin and ceftazidime/heparin solutions are colourless at the concentrations used, blinding of patients and investigators could therefore be guaranteed.
Sample size considerations
Based on a 66% success rate (34% treatment failure) in the placebo group1 and an 83% success rate (17% treatment failure) in the AB-lock group, and assuming the exponential distribution for time to ‘forced’ removal, the sample size was calculated as 152 patients in total for the study to have an 80% power to detect a 50% reduction (from 34% to 17%) of treatment failures in the AB-lock group, with a P value of 0.05 (one-sided). A one-sided approach was considered appropriate.
Statistical analysis
Analysis was conducted following the modified intention-to-treat principle, and included all randomized patients who received at least one AB-lock. The primary analysis consisted of the comparison of time to treatment failure (the primary endpoint) between placebo and AB-lock arms. The stratified logrank test was used at 0.05 (one-sided) significance level and a Kaplan–Meier survival curve comparing placebo and treatment was constructed. A one-sided approach was deemed appropriate because an increase in treatment failure as the consequence of AB-lock use is conceptually incomprehensible. As the strata, the type of bacteraemia at entry (Gram-positive, Gram-negative) was used. A multivariate analysis was performed using Cox's proportional hazard model, using the following covariates: treatment group, LTID used for total parenteral nutrition, bacterial species (coagulase-negative staphylococci, other Gram-positive bacteria, Gram-negative bacteria), catheter type (subcutaneously tunnelled or totally implanted port), neutropenia (<500 cells/mm3). Fisher's exact test was used for analysis of data in a 2 × 2 contingency table.
The statistical package SAS (version 8.2) was used for analysis. The Kaplan–Meier curve was obtained with Splus 2000. Analysis was performed independently at the Biostatistical Center, School of Public Health, Catholic University Leuven, Belgium.
Results
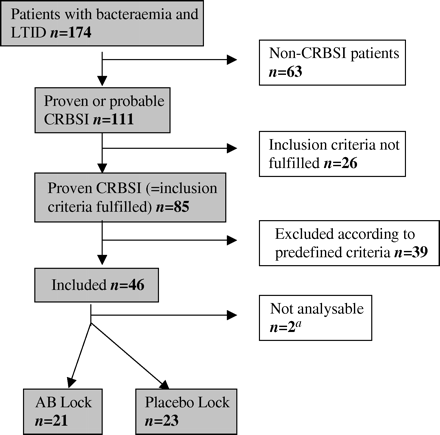
For 30 consecutive months, 174 patients with an LTID and bacteraemia were evaluated for inclusion. Eighty-five fulfilled inclusion criteria and were therefore considered to have a CRBSI. Only 46 of them could be included in the study (Figure 1). Six had a tunnelled LTID and 40 a totally implanted port. Thirty-four had a DTTP > 2 h as the inclusion criterion and 10 had bacteraemia immediately following a flush of the catheter as the inclusion criterion. In none of these 10 was the infusate a blood or platelet transfusion. For two paediatric patients, the third inclusion criterion was used and both had multiple positive blood cultures with coagulase-negative staphylococci.
The most frequently encountered reasons for exclusion were: (1) LTID not vacant for >8–12 h per day for the AB-lock (n=10); (2) yeast infection or mixed Gram-positive/negative infections (n =13); (3) CRBSI that started <14 days after insertion or pocket/tunnel infection (n=10); or (4) catheter removal preferred by the treating physician (n=7). No patient had to be excluded because the microorganism was resistant to vancomycin or ceftazidime. Two patients received no AB-lock because the LTID was shown to be occluded at the time the AB-lock (or placebo) was available for administration. The study was discontinued after 30 months of recruitment because, despite the large number of LTID being inserted in the participating hospitals (1500 per year), and notwithstanding several attempts to increase the inclusion rate, an additional 90 months would have been needed for full recruitment. As a result, 44 patients were available for the modified intention-to-treat analysis. Twenty-seven had coagulase-negative staphylococci, seven had other Gram-positive microorganisms (one Staphylococcus aureus, one Enterococcus spp., two streptococci, two corynebacteria and one Bacillus cereus) and 10 Gram-negative bacteria as the cause of CRBSI. Table 1 illustrates that the baseline characteristics of the included patients were equally divided between the groups. Only three patients were neutropenic (two in the placebo group). The median duration of the AB-lock instillation was 20 h/day for a total of 11 days.
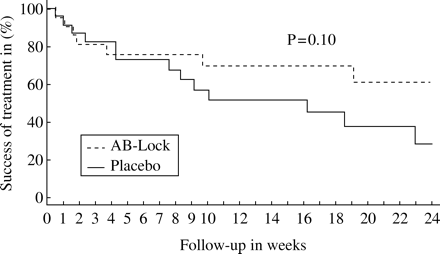
On study day 180 by Kaplan–Meier time-to-treatment failure analysis, failure to cure the CRBSI or relapse with the same strain (the primary endpoint) occurred in 20 of 44 patients (45.5%); 33% (seven of 21) in the AB-lock and 57% (13 of 23) in the placebo arm (hazard ratio 0.55, P =0.10); see Figure 2. A multivariate analysis did not show total parenteral nutrition, bacterial species (coagulase-negative staphylococci, other Gram-positive bacteria, Gram-negative bacteria), catheter-type (tunnelled or port) and neutropenia to be related to outcome.
Of the 20 failures that were observed throughout the study, 12 were considered failures because a bacteraemia with the same strain recurred after antibiotic treatment was stopped (9/23 in the placebo arm and 3/21 in the AB-lock arm, P=0.06, Fisher's exact test). Five others were failures as the catheter had to be removed for ongoing fever after >96 h of antibiotic treatment (three in the AB-lock arm and two in the placebo arm). Two failures were the result of catheter removal for a suspected CRBSI with a new strain (one confirmed CRBSI) and one because the patient died on day 5 after inclusion. No case of catheter occlusion was observed during the study.
Discussion
This study showed that in patients with an LTID-related bloodstream infection the use of an AB-lock, in addition to parenterally administered antibiotics, reduced the failure to cure the CRBSI from 57% to 33%. However, this difference was not significant (P=0.10). As described in the Results section, patient recruitment was more difficult than anticipated, for several reasons. As a result, the study was stopped prematurely after 30 months of enrolment. For that reason, the sample size was too small for this study to have sufficient statistical power to draw definite conclusions. The results are, however, in concordance with previous observational reports on the use of AB-lock for the treatment of CRBSI and give further support to the recommendation in the IDSA guidelines on this topic.1 In a secondary analysis, in which only relapse of bacteraemia with the same strain was considered a failure, fewer relapses were observed in the AB-lock arm than in the placebo arm (three of 21 versus nine of 23, P =0.06, Fisher's exact test). These data taken together therefore suggest that an AB-lock can prevent relapses of CRBSI with the same strain, but it does not increase the short-term (2 weeks) cure rate of the first episode of CRBSI. Because CRBSI tends to relapse more often in totally implanted ports than in transcutaneous central venous catheters, the use of an AB-lock might be of particular interest in patients with a totally implanted port.8
We think that several observations made during the course of this study might help the design and conduct of future studies on antibiotic or antiseptic locks for the treatment of CRBSI. An AB-lock time of at least 8 h was considered necessary for inclusion, to make sure the amount of time that any antibiotic was inside the catheter (whether as a part of the systemic antibiotic treatment or the AB-lock) would be sufficiently different between study groups. As a result of this, many patients needed to be excluded, as the catheter was not available for this period. Therefore, future studies should try to evaluate shorter lock dwell times. A recent in vitro AB-lock evaluation of linezolid, eperezolid and vancomycin suggested that linezolid and eperezolid were faster-acting AB-lock solutions than vancomycin. However, the shortest dwell-time evaluated in this study was still 24 h.9 Raad et al.10 recently demonstrated the superiority of minocycline/EDTA locks for the treatment of CRBSI in an animal model, but all locks were used continuously for 4 days. More in vitro data are needed on the speed of action of different antimicrobial locks (whether antibiotic or antiseptic) to be able to select the fastest-acting lock solution for future study.
Also, many patients were excluded because a mixture of Gram-positive and -negative microorganisms or yeasts caused the CRBSI. A broad-spectrum, non-toxic antiseptic lock compatible with silicone or polyurethane catheters might avoid this problem. Recently, ethyl-alcohol was suggested for this purpose as it is not toxic when used in small quantities and was recently shown to be compatible with polyurethane and silicone catheters.11,12 Also a taurolidine- and citrate-based lock-solution might be useful in this regard.13
Finally, we decided to only include patients with a definite CRBSI. This decision was taken because the erratic inclusion of a significant number of patients with a non-CRBSI would diminish the observed effectiveness of the AB-lock considerably, as one cannot expect to observe differences in outcome between AB-lock and placebo for non-CRBSI patients. This eventually led to the exclusion of many patients with, for example, coagulase-negative staphylococcal bacteraemia without a source (except for the LTID) when no peripheral blood culture (and therefore no DTTP) was available. An alternative approach that might be considered in future studies is the inclusion of these patients on condition that they are not neutropenic and that a minimal investigation, including a urine culture and chest X-ray, is unrevealing. The need for simpler recruitment is illustrated by the following example. Only an estimated 0.2 CRBSI per 1000 catheter days can be expected for totally implanted ports.14 When the catheterization time is 365 days, 900 000 catheter days are needed to observe 180 CRBSI. The inclusion and exclusion criteria used in this study allowed us to include 41% of the probable CRBSI. Therefore 2 200 000 catheter days or 6000 patients are needed to recruit 180 CRBSI patients. When also probable cases are included, recruitment could increase from 41% to 55% (4500 observed patients instead of 6000). For tunnelled catheters, the data might be comparable because the higher incidence is, unfortunately, compensated for by the shorter catheterization time and the tendency for earlier removal of these catheters when they are infected.
Only one CRBSI with S. aureus could be included in this study because all participating clinicians considered catheter removal the standard-of-care in this setting. The same is true for CRBSI with candidaemia. Therefore it is unlikely that a clinical trial will ever show an AB-lock to be of particular use in this subset of more aggressive infections.
Hopefully, in the near future, the prophylactic use of newer antiseptic locks (among other interventions in development) will demonstrate their value, and so the question of whether or not to use therapeutic AB-locks might become less controversial. In the intervening time, and while waiting for more evidence, this study supports the use of AB-locks for the treatment of CRBSI.
Disposition of patients evaluated for the antibiotic-lock study. LTID, long-term intravascular device, whether a tunnelled central venous catheter or a totally implanted port; CRBSI, catheter-related bloodstream infection. aIn two patients, a complete thrombotic occlusion of the catheter was observed at day 0 (after randomization but before any lock could be given).
Baseline characteristics of included patients
| . | AB-lock n=22 . | Placebo n=24 . | ||
|---|---|---|---|---|
| Age | 49 | 47 | ||
| Type of cathetera | 20/2 | 20/4 | ||
| Underlying diseaseb | 16/6 | 18/6 | ||
| Temperature (°C) | 39.0 | 38.9 | ||
| Infecting microorganism | ||||
| GP/GN | 18/4 | 18/6 | ||
| CNS | 14 | 15 | ||
| streptococci | 2 | 1 | ||
| S. aureus | 0 | 1 | ||
| Corynebacterium spp. | 1 | 1 | ||
| Bacillus cereus | 1 | 0 | ||
| E. coli or Klebsiella spp. | 2 | 2 | ||
| Agrobacterium | 0 | 1 | ||
| Stenotrophomonas | 0 | 1 | ||
| Serratia | 0 | 1 | ||
| Acinetobacter | 1 | 1 | ||
| Enterobacter | 1 | 0 | ||
| . | AB-lock n=22 . | Placebo n=24 . | ||
|---|---|---|---|---|
| Age | 49 | 47 | ||
| Type of cathetera | 20/2 | 20/4 | ||
| Underlying diseaseb | 16/6 | 18/6 | ||
| Temperature (°C) | 39.0 | 38.9 | ||
| Infecting microorganism | ||||
| GP/GN | 18/4 | 18/6 | ||
| CNS | 14 | 15 | ||
| streptococci | 2 | 1 | ||
| S. aureus | 0 | 1 | ||
| Corynebacterium spp. | 1 | 1 | ||
| Bacillus cereus | 1 | 0 | ||
| E. coli or Klebsiella spp. | 2 | 2 | ||
| Agrobacterium | 0 | 1 | ||
| Stenotrophomonas | 0 | 1 | ||
| Serratia | 0 | 1 | ||
| Acinetobacter | 1 | 1 | ||
| Enterobacter | 1 | 0 | ||
Totally implanted ports/tunnelled catheters.
Haemato-oncology/other.
GP/GN, Gram-positive/Gram-negative microorganisms; CNS, coagulase-negative staphylococci.
Baseline characteristics of included patients
| . | AB-lock n=22 . | Placebo n=24 . | ||
|---|---|---|---|---|
| Age | 49 | 47 | ||
| Type of cathetera | 20/2 | 20/4 | ||
| Underlying diseaseb | 16/6 | 18/6 | ||
| Temperature (°C) | 39.0 | 38.9 | ||
| Infecting microorganism | ||||
| GP/GN | 18/4 | 18/6 | ||
| CNS | 14 | 15 | ||
| streptococci | 2 | 1 | ||
| S. aureus | 0 | 1 | ||
| Corynebacterium spp. | 1 | 1 | ||
| Bacillus cereus | 1 | 0 | ||
| E. coli or Klebsiella spp. | 2 | 2 | ||
| Agrobacterium | 0 | 1 | ||
| Stenotrophomonas | 0 | 1 | ||
| Serratia | 0 | 1 | ||
| Acinetobacter | 1 | 1 | ||
| Enterobacter | 1 | 0 | ||
| . | AB-lock n=22 . | Placebo n=24 . | ||
|---|---|---|---|---|
| Age | 49 | 47 | ||
| Type of cathetera | 20/2 | 20/4 | ||
| Underlying diseaseb | 16/6 | 18/6 | ||
| Temperature (°C) | 39.0 | 38.9 | ||
| Infecting microorganism | ||||
| GP/GN | 18/4 | 18/6 | ||
| CNS | 14 | 15 | ||
| streptococci | 2 | 1 | ||
| S. aureus | 0 | 1 | ||
| Corynebacterium spp. | 1 | 1 | ||
| Bacillus cereus | 1 | 0 | ||
| E. coli or Klebsiella spp. | 2 | 2 | ||
| Agrobacterium | 0 | 1 | ||
| Stenotrophomonas | 0 | 1 | ||
| Serratia | 0 | 1 | ||
| Acinetobacter | 1 | 1 | ||
| Enterobacter | 1 | 0 | ||
Totally implanted ports/tunnelled catheters.
Haemato-oncology/other.
GP/GN, Gram-positive/Gram-negative microorganisms; CNS, coagulase-negative staphylococci.
We thank the following departments, physicians, nurses and patients for their participation. Dep. of Internal Medicine and Infectious Diseases (P. Demunter, H. Ceunen and M. Lejeune). Dep. of Medical Oncology, UZ Gasthuisberg Leuven (A. Van Oosterom, H. Dumez, J. Thomas, R. Paridaens). Dep. of Digestive Oncology, UZ Gasthuisberg Leuven (E. Van Cutsem). Department of Haematology, UZ Gasthuisberg Leuven (J. Maertens, M. Delforge, M. Boogaerts). Department of Pediatric Oncology, UZ Gasthuisberg Leuven (A. Uyttebroeck, M. Renard). Department of Pediatric Haematology, UZ Gasthuisberg Leuven. Department of Gastroenterology, UZ Gasthuisberg Leuven (M. Hiele, G. Van Assche). Department of Nephrology, UZ Gasthuisberg Leuven (B. Maes, E. Ghijsels). The Catheter Care Team (M. Vrebos), UZ Gasthuisberg Leuven Department of Nephrology, Virga-Jesse Ziekenhuis (J. Vanwalleghem). Department of Haematology, Virga-Jesse Ziekenhuis. Department of Nephrology, Imelda Ziekenhuis Bonheiden. All the patients that participated in this trial. GSK Belgium for providing vancomycin and ceftazidime for the preparation of the antibiotic-locks free of charge.
References
Mermel, L., Farr, B., Sherertz, R. et al. (
Carratala, J., Niubo, J., Fernandez-Sevilla, A. et al. (
Henrickson, K., Axtell, R., Hoover, S. et al. (
Schwartz, C., Henrickson, K., Roghmann, K. et al. (
Messing, B., Peitra-Cohen, S., Debure, A. et al. (
Blot, F., Nitenberg, G., Chachaty, E. et al. (
Anthony, T. & Rubin, L. (
Flynn, P. M., Willis, B., Gaur, A. H. et al. (
Curtin, J., Cormican, M., Fleming, G. et al. (
Raad, I., Hachem, R., Tcholakian, R. et al. (
Dannenberg, C., Bierbach, U., Rothe, A. et al. (
Aiyangar, A., Crone, W., Crnich, C., et al. (
Shah, C., Mittelman, M., Costerton, J. et al. (
Author notes
1Internal Medicine and Infectious Diseases, Erasmus MC, Rotterdam, The Netherlands; 2 Internal Medicine and Infectious Diseases, UZ Gasthuisberg, Leuven; 3 Oncological Surgery, UZ Gasthuisberg, Leuven, Belgium